Allergic conjunctivitis (AC) is one of the most common allergic ocular diseases worldwide. Osteopontin (OPN), as a recently described Th2 inflammation related protein, may play a role in the pathogenesis of AC. The aim of this study was to identify the expression of OPN in children with AC.
MethodsEighty AC children (seasonal and perennial AC) and twenty controls were enrolled in this study. Serum and tears of different time points (during and out of the pollen season) were collected and used for enzyme-linked immunosorbent assay (ELISA) of OPN and T-help cell related cytokines, respectively. The relationship between serum and tears OPN and Th1/2/17Treg related cytokines as well as disease severity were analysed.
ResultsOur results showed that expression of tear OPN protein by perennial AC patients increased significantly compared with controls or seasonal AC patients out of the pollen season. Tear OPN expression was positively related to local Th2/17 cytokines and negatively related to IL-10 and TGF-β expression. The tear OPN expression was also significantly related to disease severity.
ConclusionTear OPN reflects the local clinical status of ocular allergy and might play an important pathophysiological role in local Th2/17/Treg inflammation in children with AC.
Allergic conjunctivitis (AC) in children is one of the most common allergic diseases worldwide and presents as ocular itching, hyperaemia, mucous discharge and tears.1 Better understanding the pathogenesis of AC may help in treating patients.2 Recently, the role of various cells and cytokines has been investigated in the immune regulation of ocular allergy.3
Osteopontin (OPN) is a phosphorylated acidic glycoprotein that can bind with certain CD44 variants and integrin receptors.4 Various cells can secrete OPN, such as osteoblasts, osteoclasts, macrophages, T-cells, hematopoietic cells, vascular smooth muscle cells, fibroblasts, myocardial cells and so on.5,6 OPN has been reported to be deeply involved in cellular migration or infiltration, restoration of tissues and inflammatory diseases.7 Recent studies show that OPN may be involved in Th2-mediated diseases.8 Taken together, OPN may play different roles under varied backgrounds due to its complicated molecular structure and receptors.
As the role of OPN in allergy (especially in asthma and allergic rhinitis) has received more and more attention, we postulated that OPN may be involved in the pathogenesis of AC. The aim of this study was to evaluate OPN expression in the AC patients and compare its differences among different groups and seasons, and analyse the relationship between OPN and disease severity.
MethodsSubjectsAll the subjects (7–18 years old) were recruited at the Department of Ophthalmology, Yi Chang Hospital of Traditional Chinese Medicine from January 2016 to October 2016. The study was performed with the approval of the local ethics committee and with the patients’ written informed consent.
Eighty AC children were diagnosed based on the slit lamp examination (conjunctival hyperaemia, follicles, and papillae) and symptoms such as itching, redness, ocular pain and tearing in children without proliferative lesions for at least one year. The eighty children were sensitised as demonstrated by a positive skin prick test (>3mm) or the detection (>0.35kIU/L) of serum specific IgE (Phadia) to common inhalant allergens (dust mites, pollens, pets, moulds, cockroach, etc.). AC children were subdivided into perennial AC (PAC) and seasonal AC (SAC) according to the duration of symptoms. Twenty healthy children without ocular or systemic diseases were enrolled as controls.
Ocular exclusion criteria were parasitic, bacterial or viral conjunctivitis, glaucoma, rosacea, dry eye syndrome, and ocular wound. Systemic exclusion criteria were infections, atopic dermatitis, eczema, nettle rash, and systemic diseases. None of the patients was receiving systemic or local steroids or anti-allergic drugs one month before sample collection or wearing contact lenses.
Disease severityOcular itching, conjunctival hyperaemia, conjunctival chemosis, eyelid swelling, and tearing were scored according to a semi-quantitative scale from 0 to 3 (0=normal, 1=mild, 2=moderate, or 3=severe) as described elsewhere.9 The clinical score was the sum of the scores for each criterion, where 0 represented no symptom/sign and 15 the maximum. For PAC patients, the symptoms were scored when the patients were enrolled. The scores for SAC patients were collected during the pollen season.
Tear and serum collectionAround 50μl of tear sample was collected as previously reported without stimulation.10 Briefly, a micro-haematocrit tube (NRIS microhematocrit tube, Herlev, Denmark) was introduced into the inferior tear meniscus and tears were collected into the plastic tube by using a bulb attached to one end of the tube without the use of saline solution. This process was duplicated two or three times at 4–6min intervals. All samples were stored at −80°C until assay and tested within 2h after thawing, followed by centrifugation. The samples were diluted 10-fold times before test.
Venous blood samples were collected into Vacuette tubes and centrifuged at 3000×g for 15min at 4°C. Serum samples were stored at −80°C. These samples and tears were used for enzyme-linked immunosorbent assay (ELISA) measurement.
The tear and blood for SAC patients were collected during the pollen season and two months before the pollen season, respectively. For PAC patients and controls, the tear and blood were taken when the patients were enrolled.
ELISA measurement for cytokinesELISA kits were used for measuring serum OPN, IL-4, IL-5, IFN-gamma, IL-10, IL-17, TGF-β (R&D systems, USA) according to the manufacturer's protocols. The detection limits of the assays were as follows: OPN, 0.312pg/mL, IL-4, 1.56pg/mL, IL-5, 7.8pg/mL, IFN-gamma, 12.5pg/mL, IL-10, 3.9pg/mL, IL-17, 15pg/mL, TGF-β, 15.4pg/mL. All patients had values above the detection limits.
Statistical analysisStatistical analysis was performed using Mann–Whitney or Wilcoxon signed rank test. The pre-test Friedman test was done for multiple comparisons. The Spearman rank correlation test was used to analyse the correlation among the expression of biomarkers. p<0.05 was considered as significant difference.
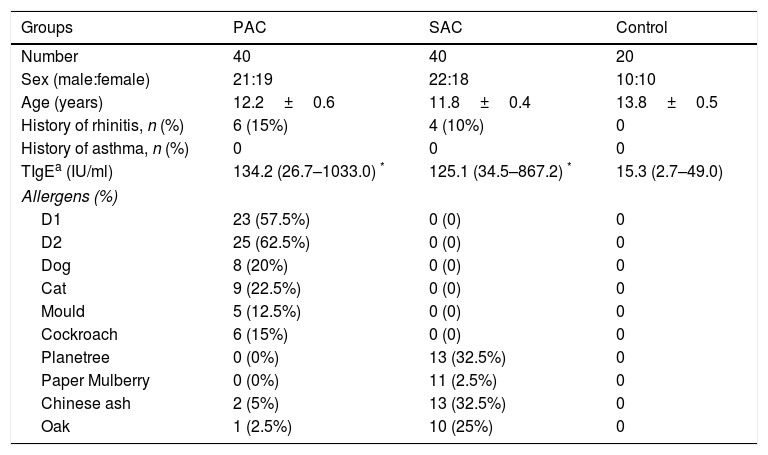
ResultsDemographic characteristics of study population and clinical outcomeThis study was conducted with 40 SAC patients, 40 PAC patients and 20 controls. The demographic characteristics of patients were summarised in Table 1. Patients from three groups have comparable sex ratio and age. Patients from both PAC and SAC groups had higher TIgE levels compared with controls.
Demographic characteristic of PAC, SAC and normal controls.
| Groups | PAC | SAC | Control |
|---|---|---|---|
| Number | 40 | 40 | 20 |
| Sex (male:female) | 21:19 | 22:18 | 10:10 |
| Age (years) | 12.2±0.6 | 11.8±0.4 | 13.8±0.5 |
| History of rhinitis, n (%) | 6 (15%) | 4 (10%) | 0 |
| History of asthma, n (%) | 0 | 0 | 0 |
| TIgEa (IU/ml) | 134.2 (26.7–1033.0) * | 125.1 (34.5–867.2) * | 15.3 (2.7–49.0) |
| Allergens (%) | |||
| D1 | 23 (57.5%) | 0 (0) | 0 |
| D2 | 25 (62.5%) | 0 (0) | 0 |
| Dog | 8 (20%) | 0 (0) | 0 |
| Cat | 9 (22.5%) | 0 (0) | 0 |
| Mould | 5 (12.5%) | 0 (0) | 0 |
| Cockroach | 6 (15%) | 0 (0) | 0 |
| Planetree | 0 (0%) | 13 (32.5%) | 0 |
| Paper Mulberry | 0 (0%) | 11 (2.5%) | 0 |
| Chinese ash | 2 (5%) | 13 (32.5%) | 0 |
| Oak | 1 (2.5%) | 10 (25%) | 0 |
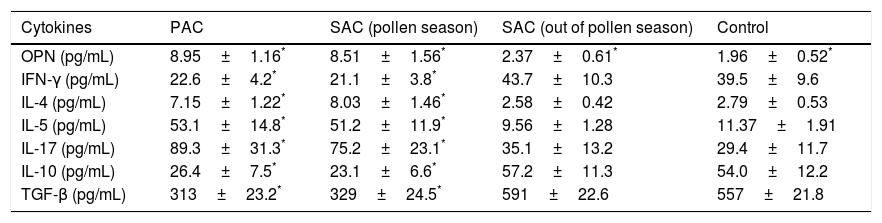
Tear IFN-gamma, IL-10 and TGF-β protein expression in PAC or SAC patients during the pollen season decreased significantly compared with controls and SAC patients out of pollen season, while tear IL-4, IL-5 and IL-17 levels in PAC or SAC patients during the pollen season increased significantly compared with controls and SAC patients out of the pollen season (Table 2). However, the serum Th1/Th2/Th17/Treg cytokines expression between different groups showed no significant differences (data not shown).
Comparison of Tear Th cytokines between AC children and normal controls.
| Cytokines | PAC | SAC (pollen season) | SAC (out of pollen season) | Control |
|---|---|---|---|---|
| OPN (pg/mL) | 8.95±1.16* | 8.51±1.56* | 2.37±0.61* | 1.96±0.52* |
| IFN-γ (pg/mL) | 22.6±4.2* | 21.1±3.8* | 43.7±10.3 | 39.5±9.6 |
| IL-4 (pg/mL) | 7.15±1.22* | 8.03±1.46* | 2.58±0.42 | 2.79±0.53 |
| IL-5 (pg/mL) | 53.1±14.8* | 51.2±11.9* | 9.56±1.28 | 11.37±1.91 |
| IL-17 (pg/mL) | 89.3±31.3* | 75.2±23.1* | 35.1±13.2 | 29.4±11.7 |
| IL-10 (pg/mL) | 26.4±7.5* | 23.1±6.6* | 57.2±11.3 | 54.0±12.2 |
| TGF-β (pg/mL) | 313±23.2* | 329±24.5* | 591±22.6 | 557±21.8 |
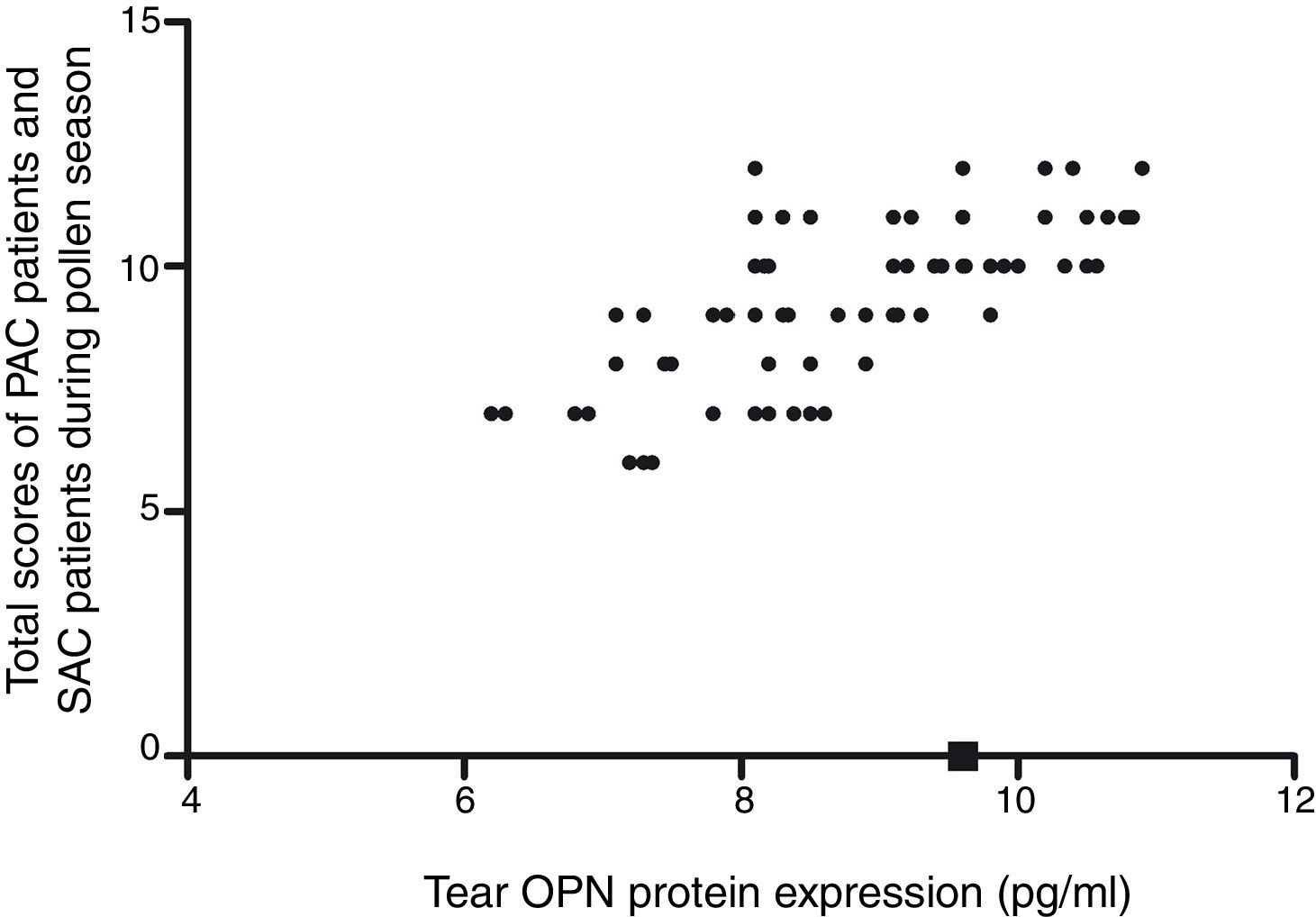
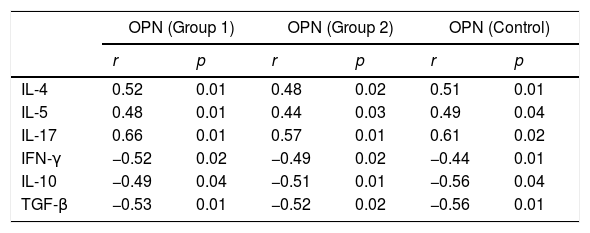
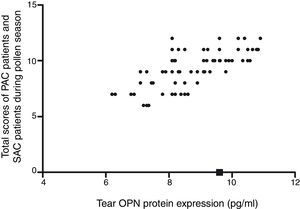
The tear OPN protein expression in PAC or SAC patients during the pollen season increased significantly compared with controls and SAC patients out of the pollen season (9.8±1.5 vs 9.3±1.5 vs. 2.6±0.9 vs. 2.3±0.7, Fig. 1). Correlation analysis showed that tear OPN expression in PAC patients or SAC (during the pollen season) patients was positively correlated to Th2/17 cytokines (IL-4, r=0.52; IL-5, r=0.61; IL-17, r=0.56) and negatively correlated to Th1/Treg cytokines (IFN-γ, r=−0.52; IL-10, r=−0.49; TGF-β, r=−0.53, Table 3). We also found that tear OPN expression in PAC patients or SAC (during the pollen season) patients were positively correlated to disease severity (Fig. 1).
Relationship between tear OPN level and Th cytokines in AC and control children.
| OPN (Group 1) | OPN (Group 2) | OPN (Control) | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| IL-4 | 0.52 | 0.01 | 0.48 | 0.02 | 0.51 | 0.01 |
| IL-5 | 0.48 | 0.01 | 0.44 | 0.03 | 0.49 | 0.04 |
| IL-17 | 0.66 | 0.01 | 0.57 | 0.01 | 0.61 | 0.02 |
| IFN-γ | −0.52 | 0.02 | −0.49 | 0.02 | −0.44 | 0.01 |
| IL-10 | −0.49 | 0.04 | −0.51 | 0.01 | −0.56 | 0.04 |
| TGF-β | −0.53 | 0.01 | −0.52 | 0.02 | −0.56 | 0.01 |
Group 1 for PAC and SAC children during pollen season, Group 2 for SAC children out of pollen season.
Generally, AC was believed to be a Th2-predominant response dominated by cytokines IL-4, IL-5, and IL-13, eosinophils, and IgE. Previous reports show that IL-4 is increased in the culture supernatants of samples obtained by brush collection in patients with AC.11 Increased transcripts of IL-4 and IL-13 were also detected by the reverse transcription (RT)-PCR method in samples from AC patients.12 However, the regulation pathway in Th2-predominant response was not fully understood.
OPN has also been detected in a variety of human body fluids including blood, urine, and milk.13 OPN, as a multifunctional protein, has been investigated in allergy and asthma only recently. Several studies have investigated the role of OPN in promoting chemotaxis inflammatory cells such as eosinophils and mast cells in asthma.14,15 For eyes, expression of OPN has been identified in the retina, and OPN-like immunoreactivity is present in the ganglion cells of rats.16 The clinical severity of allergic conjunctival diseases was also found to be correlated significantly with the level of OPN.10 However, few studies have examined the relationship between OPN and AC in Chinese.
Our results showed that the tear OPN protein expression in PAC or SAC children during the pollen season increased significantly compared with controls and those SAC patients out of the pollen season. OPN was also found to be positively related to expression of Th2/17 cytokines. However, the relationship was not very strong (IL-4, r=0.52; IL-5, r=0.48). These results suggested that OPN may play a partial role in Th2 regulation and other factors also contributed to Th2 regulation, since allergic diseases are very complex process. Consistent with our study, two studies performed by Liu's group showed that OPN expression was enhanced in Th2 disease of Chinese population (nasal polyps and allergic rhinitis), suggesting that OPN may enhance Th2 response.17,18 Our study also provides evidence that OPN may be involved in Th17 response in AC, which has not been reported previously. Treg related cytokines included IL-10 and TGF-β. IL-10 can inhibit synthesis and function of both Th1 and Th2 inflammatory cytokines.19 TGF-β is believed to be important in the regulation and differentiation of Foxp3+ Regulatory T cells, which can suppress immune reactions.19 Previous studies20,21 reported an increase in TGF-β in allergic rhinitis or asthma related to mucosal remodelling. On the contrary, several studies22,23 showed that serum TGF-β levels were significantly lower in allergic rhinitis and immunotherapy can induce expression of TGF-β.24 Our results showed that tear TGF-β level in PAC or SAC patients during the pollen season decreased significantly, suggesting that local TGF-β in AC may play a pro-inflammatory effect. However, the exact mechanisms of TGF-β in AC need further exploration. Our data also showed that tear OPN may be related to IL-10 and TGF-β expression and thus may regulate imbalance of Th cells during AC.
In this cross-sectional study, a statistical association between OPN and other cytokines was found. However, our results cannot provide a confirmative causality between OPN and other cytokines. The effect between OPN and other cytokines may be unidirectional or bidirectional, or they just follow parallel paths due to another common cause acting on both. Therefore, the role of OPN in Th response needs to be further explored by more in vivo and in vitro studies.
Interestingly, the correlation of OPN and Th cytokines in tears was not found in serum in this study. Besides, the serum OPN and Th1/Th2/Th17/Treg cytokines level were also not different among groups, suggesting that OPN may only exert its role locally. Consistently, the local inflammation in the AC may also be observed in the development of allergic rhinitis and asthma.25,26 These results confirmed the importance of local inflammatory reaction in allergic diseases.
Correlation analysis also showed that tear OPN expression was positively related to disease severity. The correlation between OPN and disease severity and high OPN expression in the allergy season suggested that OPN may be served as a possible biomarker to use in differential diagnosis of other disorders, to monitor the activity of the disease or the response to therapy. However, whether OPN may be used as a biomarker in clinic needed further exploration. Collectively, our finding showed that during AC, OPN expression was increased and this up-regulation of OPN may be related to high Th cytokines production. We also found that tear OPN level was correlated to disease severity. However, the exact mechanism of OPN expression during AC is still largely unknown and needs further exploration.
Conflicts of interestThe authors have no other funding, financial relationships or conflicts of interest to disclose.
FundingNone.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.