Desensitisation or specific oral tolerance induction (SOTI) to food is a new topical-therapeutic approach of food allergy for those children who have not achieved tolerance spontaneously. The objective of this study is to induce clinical tolerance in children with persistent allergy using an oral desensitisation protocol with powdered pasteurised egg.
MethodsSeventy-two patients with egg allergy confirmed by open oral challenge test were randomly assigned to SOTI or elimination diet as a control group. Forty children (5–15 years) underwent a SOTI beginning with 1mg and increasing the dosage weekly until a dose of 10g, equivalent to an egg. The control group included 32 patients (4–15 years).
ResultsThe procedure's average duration was 10 weeks (range 4–28 weeks). Three patients were withdrawn from the protocol for persistent gastrointestinal symptoms. During SOTI, 21 children (52.5%) presented symptoms. In eight the symptoms were mild and required no treatment. In the other 13 (61.90%), the reactions were more severe. Seventeen children finished the treatment over a year ago and 20 in the past 6–12 months. Thirty-seven patients (92.5%) in the active group achieved tolerance to egg, versus 21.8% in the control group. We only found statistically significant differences (p<0.05) for skin prick tests with powdered egg at various dilutions and IgG levels with egg white after SOTI. Specific IgE concentration did not change significantly.
ConclusionsOur SOTI protocol is a safe, effective treatment for food allergy and of reasonable duration, confirming that tolerance can be induced in children who have not achieved it spontaneously.
Egg allergy is the most common cause of food allergies in children after 1 year of age.1,2 Egg white, due to its higher protein content, causes most sensitisations and clinical manifestations.
The estimated prevalence of allergy to eggs is between 0.5 and 2.7% of the overall population in the first few years of life, although sensitivity to eggs is expressed only through skin tests and in the laboratory results can rise to as much as 5% of the population.3
In the majority of children who are allergic to eggs, the problem subsides, so by the age of 4–5 years, 57% of children with egg allergy achieve spontaneous tolerance.3–5 From then on, the likelihood of spontaneous occurrence of tolerance is diminished and it is often the case that severe clinical reactions are sparked from even the slightest traces of the food.
Until now, the usual recommended treatments have been exclusion diets and awareness-building of the patient and family members about the exclusion diet and possible latent sources, in order to avoid accidental intake.
With the aim of modifying the natural history of food allergies, various therapeutic alternatives have been considered, such as immunotherapy or anti-IgE therapy.6,7 When it is a matter of a food allergen that is consumed on a daily basis, as is the case with eggs, the psycho-emotional and economical impact on patients and their families is huge, significantly affecting their quality of life.8
In response to this situation, other alternatives have been sought, such as desensitisation or specific oral tolerance induction (SOTI) to the food.5,7,9–21 In recent years, a number of oral tolerance induction series in patients allergic to cow's milk have been reported in journals. However, when it comes to egg, the experience is not so extensive and in some studies anaphylactic patients have been excluded. In 2007, Buchanan et al.12 published a tolerance induction protocol based on their experience with seven children. Itoh et al.16 have recently communicated a rush SOTI protocol successfully tested with six patients. Furthermore, Vickery et al.17 have communicated a sensibly longer protocol, which controls the progress of the patients with specific IgE levels. More recently, García Rodríguez et al.18 have published their experience with a larger cohort (23 patients) who underwent a 5-day rush SOTI protocol.
The main objective of this study is to induce clinical tolerance in children with persistent allergy using an oral desensitisation protocol with powdered pasteurised egg. When total tolerance is not attained, we aim to raise the tolerance threshold in order to avoid potentially serious adverse reactions from accidental intake.
Materials and methodsSeventy-two children with persistent egg allergy between the ages of 4 and 15 years were followed. Patients were randomly assigned to SOTI (treatment Group A) or the elimination diet (control Group B).
A tolerance induction protocol was applied to patients in Group A. These patients were included from February 2007 to January 2010.
Prior to inclusion in the desensitisation protocol, the clinical history records and immunological analysis were reassessed and an oral challenge test with egg was carried out on those who had not suffered clinical episodes within the previous 3 months.
The oral challenge tests were performed in the form of open oral challenge tests, preceded in some cases by cutaneous and/or labial contact tests.
Before and after the procedure, and in the follow-ups at 6 and 12 months, prick tests with powdered egg at various dilutions (10mg/ml, 5mg/ml, and 1mg/ml) were carried out, and IgG and IgE were measured both in total and in specific amounts for egg white, yolk, ovoalbumin (OVA) and ovomucoid (OVM) (CAP-Phadia, Uppsala, Sweden).
For the positive and negative test controls, histamine phosphate (10mg/ml) and saline solution, respectively, were used. A reading was taken after 15min, and the cutaneous test was considered positive when the size of the swelling was equal or superior to 3mm compared to the negative control.
Cutaneous tests with freeze-dried powdered egg were carried out on 10 control patients allergic to egg, aiming to obtain a size of wheal similar to that obtained with commercial egg extract (Laboratorios Leti, Barcelona, Spain and ALK-Abelló, Madrid, Spain).
Medical data were gathered, including initial severity of the allergy; sex; age; accidental reactions; symptomatology with the provocation test; dosage tolerance in the clinic and at home; as well as symptoms and treatment if required.
Patient characteristicsSeventy-two children were followed. Forty children were randomised in the Group A (SOTI group) and 32 patients in the elimination diet group (Group B).
All the patients tested positive to cutaneous tests and specific IgE tests to egg and its fractions (white, yolk, OVA, and OVM).
Group A patients had been diagnosed of egg allergy at a mean age of 11.5 months (range 2–20 months). For all patients, an open oral challenge test with egg was carried out beginning with doses of between 1/32 and 1/16 of egg and gradually increasing doses every 30min until symptoms appeared.
Seven patients in Group A presented anaphylactic reactions, requiring treatment with epinephrine in six cases. Nine patients suffered urticaria/angio-oedema; five had digestive symptoms (in two cases, severe abdominal pain requiring corticosteroid treatment). The remaining six patients had oral allergy syndrome which was treated with antihistamines.
In 13 patients this oral challenge test was not carried out due to recent clinical manifestation following accidental intake in the 3 months leading up to the beginning of the treatment.
Before starting the treatment 21 of the 40 patients (52.5%) had presented symptoms due to accidental egg intake and 15 of these on more than one occasion. Fifty percent of the patients presented allergies to other foods, 70% had asthma and 92.5% had atopic dermatitis.
The 32 patients included in the control Group (B) had been diagnosed of egg allergy at a mean age of 11.4 months (range 4–24 months). Nine out of 32 patients had an anaphylactic reaction during the open oral challenge test.
Desensitisation protocolA protocol with powdered pasteurised egg mixed with juice or milkshakes was established enabling patients to continue treatment at home, with periodic weekly visits to the outpatient allergy department.
For all of the patients, informed consent, approved by the ethical committee of the hospital, was obtained from the parents. The parents were trained in the recognition and treatment of possible adverse reactions that could present.
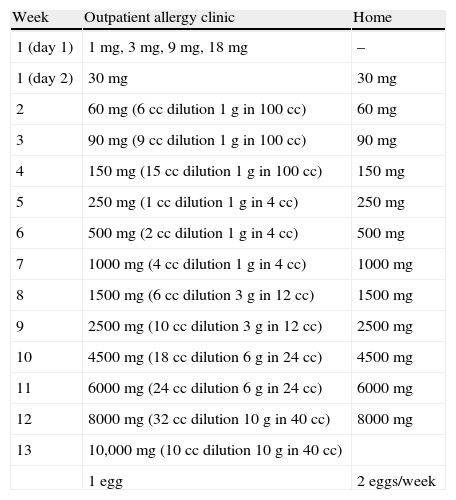
On the first day, fractionated doses were administered until reaching 31mg of egg, beginning with 1mg and continuing with 3, 9, and 18mg at 30min intervals. On the second day, 30mg in one single dose was administered, with the treatment continuing at home at this same dosage. Subsequently, weekly increases were made in the clinic until 10g of powdered egg, the equivalent of one egg, was reached. Lastly, tolerance of the natural food was checked (Table 1).
Desensitisation protocol and administered dose.
| Week | Outpatient allergy clinic | Home |
| 1 (day 1) | 1mg, 3mg, 9mg, 18mg | – |
| 1 (day 2) | 30mg | 30mg |
| 2 | 60mg (6cc dilution 1g in 100cc) | 60mg |
| 3 | 90mg (9cc dilution 1g in 100cc) | 90mg |
| 4 | 150mg (15cc dilution 1g in 100cc) | 150mg |
| 5 | 250mg (1cc dilution 1g in 4cc) | 250mg |
| 6 | 500mg (2cc dilution 1g in 4cc) | 500mg |
| 7 | 1000mg (4cc dilution 1g in 4cc) | 1000mg |
| 8 | 1500mg (6cc dilution 3g in 12cc) | 1500mg |
| 9 | 2500mg (10cc dilution 3g in 12cc) | 2500mg |
| 10 | 4500mg (18cc dilution 6g in 24cc) | 4500mg |
| 11 | 6000mg (24cc dilution 6g in 24cc) | 6000mg |
| 12 | 8000mg (32cc dilution 10g in 40cc) | 8000mg |
| 13 | 10,000mg (10cc dilution 10g in 40cc) | |
| 1 egg | 2 eggs/week |
All the patients attended the clinic every time the dosage was increased and stayed under observation for at least an hour. The children continued taking the last tolerated amount at home on a daily basis. If the tolerance at home was not total, they either continued with a smaller dose or repeated the same quantity. Their family members were trained to recognise possible adverse reactions that could arise and treat them with antihistamines and intramuscular epinephrine.
The procedure was initially programmed to last for 13 weeks. Antihistamines, bronchodilators or anti-inflammatory drugs were only administered if they were required for the treatment of adverse reactions or if rhinitis, atopic dermatitis or asthma comorbidity were present.
A month after finishing the treatment the patients were contacted by telephone and if they had good tolerance, they were recommended a normal (non egg-free) diet. The patients had follow-ups at the clinic 6 and 12 months after achieving tolerance.
An open oral challenge with raw egg white was performed in patients in Group A at least 6 months after finishing SOTI.
Statistical analysisStatistical analysis was carried out using SPSS Version 18 (SPSS Inc., Chicago, IL, USA). For description of quantitative variables, the median and interquartile range (IQR) were used. The means of non-normally distributed variables (SPT results and IgE and IgG levels before and after desensitisation and at 6- and 12-month follow-ups) were compared using the Wilcoxon test. The differences were considered significant when the P-value was less than 0.05.
ResultsForty patients (30 boys and 10 girls) were included in the active Group (A), whose mean age was 8.7 (range 5–15). The mean values of specific IgE for egg white and OVM were 9.03kU/l and 7.98kU/l, respectively (0.35–100kU/l for both).
Mean size of the wheal after a prick test with egg white and OVM was 8.74mm and 8.35mm respectively (4–16mm). Mean size of the wheal after a prick test with egg at 10, 5, and 1mg/ml concentrations was 10.31mm, 8.64mm, and 6.92mm respectively (range 10mg/ml 4–20mm, 5mg/ml 4–15mm and 1mg/ml 2–15mm).
In 20 patients the established protocol was followed and in the remaining 20 the procedure was begun with higher doses as the children had shown higher thresholds in the challenge tests.
The average duration of the procedure was 10 weeks (ranging from 4 to 28 weeks). Some 92.5% of the children (37 patients) achieved tolerance of a whole egg (10g).
Two patients were withdrawn (patients 2 and 39) from the study due to repeated digestive symptoms with the first doses. Another patient (patient 16) was withdrawn with 500mg due to suspected and later confirmed eosinophilic oesophagitis.
One patient suffered various anaphylactic reactions during the procedure, both in the clinic and at home, which were controlled with epinephrine and led to the treatment being continued with well-cooked egg, of which there was good tolerance.
Nine of the patients had previously successfully undergone an oral tolerance induction protocol with milk.
During the desensitisation period, 21 of the patients (52.5%) had symptoms at some stage of the dosage. In eight of the children the symptoms were very mild and did not require treatment. In the 13 other patients (61.90%) the reactions were more severe, resulting in doses having to be repeated, and in five cases epinephrine was needed (Table 2).
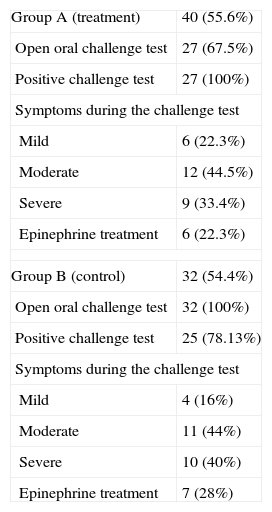
Open oral challenge outcome.
| Group A (treatment) | 40 (55.6%) |
| Open oral challenge test | 27 (67.5%) |
| Positive challenge test | 27 (100%) |
| Symptoms during the challenge test | |
| Mild | 6 (22.3%) |
| Moderate | 12 (44.5%) |
| Severe | 9 (33.4%) |
| Epinephrine treatment | 6 (22.3%) |
| Group B (control) | 32 (54.4%) |
| Open oral challenge test | 32 (100%) |
| Positive challenge test | 25 (78.13%) |
| Symptoms during the challenge test | |
| Mild | 4 (16%) |
| Moderate | 11 (44%) |
| Severe | 10 (40%) |
| Epinephrine treatment | 7 (28%) |
The three patients who were withdrawn (patients 2, 16, and 39) from the procedure presented abdominal pain and repeated vomiting that required corticosteroid and antihistamine treatment (Table 3).
Adverse reactions during the SOTI.
| Patient no. | Age at start of protocol (years) | Allergen-specific IgE (kU/l) | Dosage | Clinical reactions | Treatment |
| 2a | 6 | Egg white>100Ovomucoid>100 | 60mg | Abdominal pain, vomiting (mild) | None |
| 5 | 6 | Egg white 8.56Ovomucoid 6.38 | 30mg, 50mg, 1g | Oropharyngeal pruritus (mild) | None |
| 8 | 7 | Egg white1.36Ovomucoid 0.35 | 30mg, 1g | Abdominal pain (mild) | None |
| 10 | 9 | Egg white 2.14Ovomucoid 0.77 | 1g | Abdominal pain | CorticosteroidAnti H1 |
| 11 | 8 | Egg white 32Ovomucoid 37.3 | 60mg, 90mg, 2g | Urticaria, cough | AdrenalinCorticosteroidAnti H1Beta 2 agonist |
| 12 | 6 | Egg white 4.04Ovomucoid 1.93 | 1g | Abdominal pain (mild) | None |
| 13 | 7 | Egg white 0.64Ovomucoid 0.40 | 2.5g | Abdominal pain, vomiting, rhinoconjunctivitis | Corticosteroid Anti H1 |
| 14 | 7 | Egg white 3.22Ovomucoid 4.75 | 1g, 1.5g | Abdominal pain (mild) | None |
| 16a | 7 | Egg white 39.6Ovomucoid 22 | 30mg, 80mg, 500mg, | Abdominal pain, vomiting | CorticosteroidAnti H1 |
| 20 | 8 | Egg white 2.16Ovomucoid 1.87 | 1g | Abdominal pain | CorticosteroidAnti H1 |
| 25 | 13 | Egg white 0.35Ovomucoid 0.35 | 400mg5g | Perioral exanthema (mild) | None |
| 26 | 9 | Egg white 7.95Ovomucoid 8.02 | 1.25g7.5g | Rhinoconjunctivitis cough | AdrenalinBeta 2 agonist |
| 27 | 12 | Egg white 36.7Ovomucoid 43 | 6.25mg7.5g | Asthma, Urticaria, Rhinoconjunctivitis | CorticosteroidAnti H1AdrenalinBeta 2 agonist |
| 28 | 15 | Egg white 17.5Ovomucoid 20.3 | 2.5g10g | Abdominal pain, diarrhoea, perioral urticaria (mild) | None |
| 30 | 10 | Egg white 42.1Ovomucoid 11 | 150mg, 250mg, 500mg | Abdominal pain, vomiting | CorticosteroidAntiH1 |
| 31 | 9 | Egg white 2.16Ovomucoid 2.36 | 500mg | Abdominal pain (mild) | None |
| 32 | 14 | Egg white 3.2Ovomucoid 2.25 | 7.5g | Vomiting | Corticosteroid Anti H1 |
| 36 | 8 | Egg white 0.77Ovomucoid 0.82 | 2.5g | Perioral urticaria | Anti H1 |
| 38 | 8 | Egg white 2.26Ovomucoid 1.65 | 1g | Vomiting (mild) | None |
| 39a | 9 | Egg white 5.8Ovomucoid 9.55 | 1g, 8g | Abdominal serious pain, cough, asthma | AdrenalinBeta 2 agonist |
| 40 | 10 | Egg white 3.98Ovomucoid 5.15 | 1g | Cough, rhinoconjunctivitis, urticaria | AdrenalinCorticosteroidAntH1Beta 2 agonist |
Anti H1, H1 antihistamine.
Thirty-seven patients completed the desensitisation treatment. Twenty out of the 37 patients (54%) had good tolerance in the open oral challenge test with raw egg white and continued a normal diet devoid of any egg-related restrictions. Five did not tolerate raw egg white and follow a raw egg-free diet.
The two patients were already referred to tolerated cooked egg and suggested to avoid the intake of raw egg. Seventeen children finished the treatment over a year ago and 20 in the last 6–12 months.
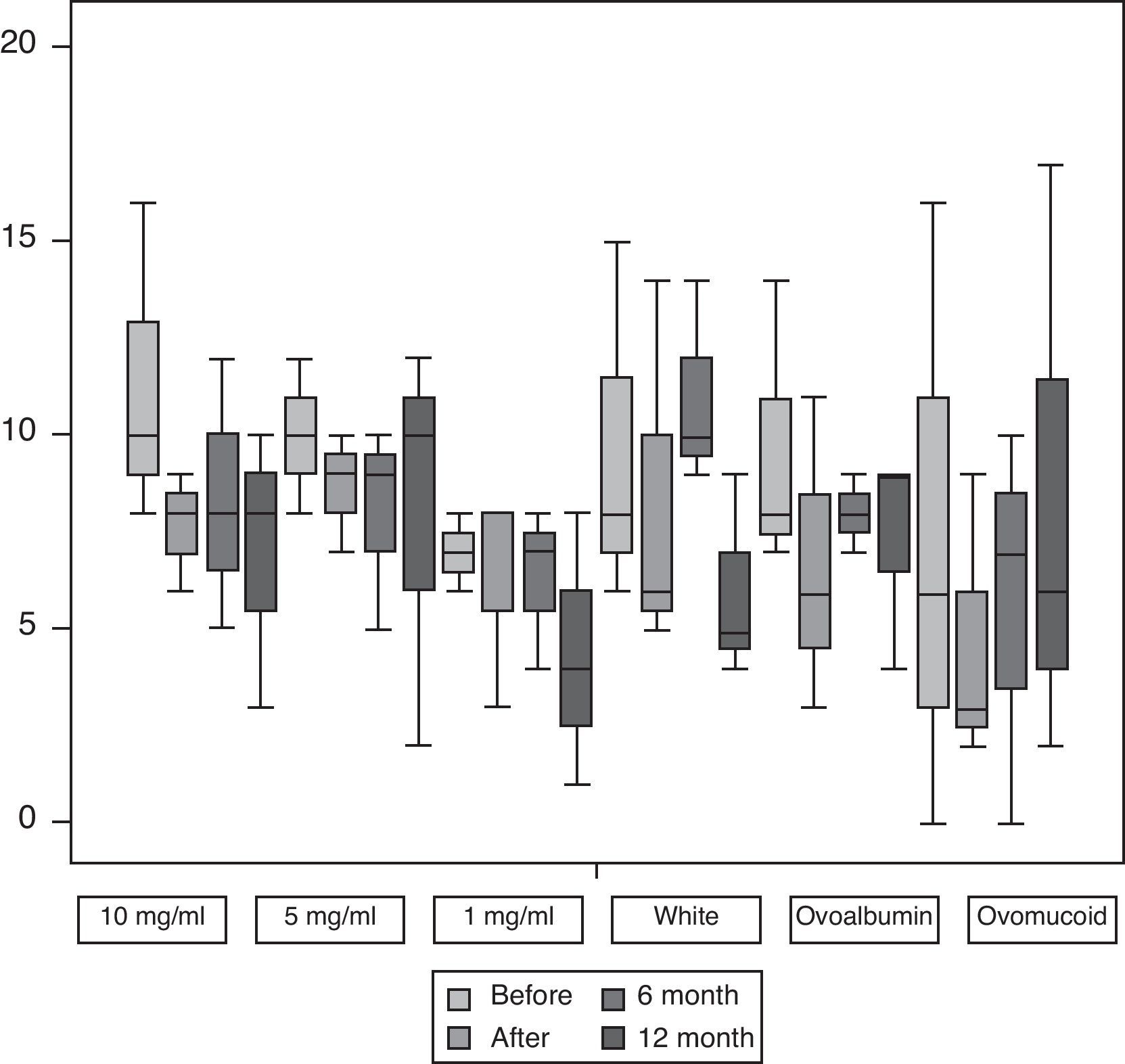
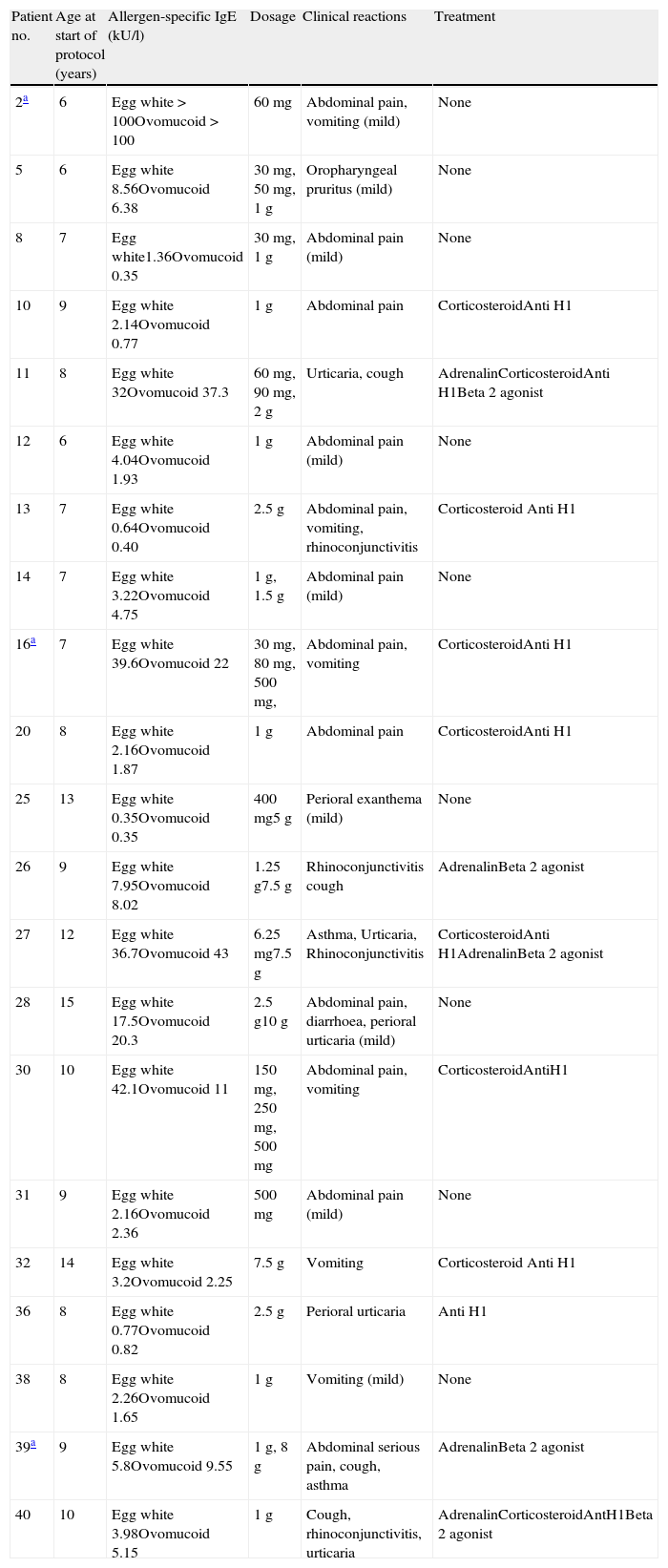
Fig. 1 shows the median and interquartile range of the greater diameter of the cutaneous tests with egg at 10mg/ml, 5mg/ml and 1mg/ml and with egg white, OVA and OVM carried out before and after SOTI and in the follow-ups at 6 and 12 months. In all the patients a reduction was observed in the size of the wheal at the end of the treatment, achieving statistical significance (p<0.001) for the cutaneous tests at all the dilutions.
Skin prick test with powdered egg at dilutions 10mg/ml, 5mg/ml, and 1mg/ml, and egg white, ovoalbumin and ovomucoid before and after SOTI and in the follow-ups at 6 and 12 months. The horizontal line in the middle of each box indicates the median, and the top and bottom borders of the box mark the 75th and 25th percentiles, respectively. The whiskers above and below the box mark the 90th and 10th percentiles, respectively.
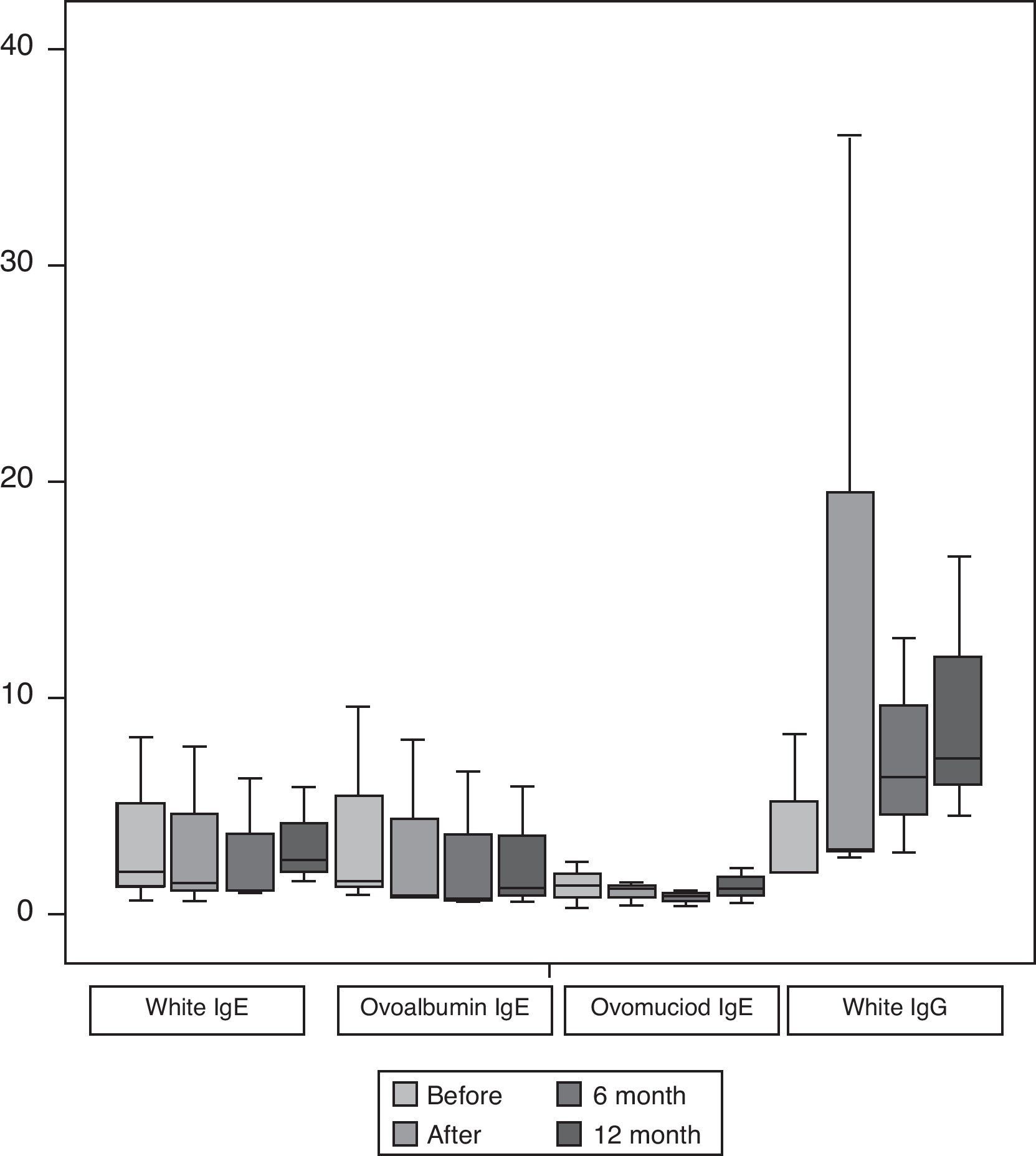
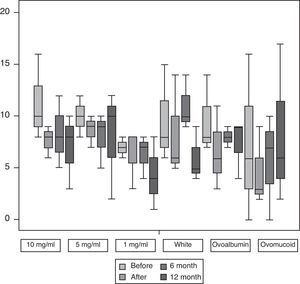
Fig. 2 shows the median and interquartile range of the results of the specific IgE for egg white, OVA and OVM expressed in kU/l as well as IgG for egg white all carried out before and after finishing the protocol and the follow-ups at 6 and 12 months, as in the case of the cutaneous tests. We only found statistically significant differences (p<0.05) for the levels of IgG with egg white at the end of the induction. We did not find statistically significant differences in the specific IgE values.
Specific IgE for egg white, ovoalbumin and ovomucoid and IgG for egg white before and after SOTI and the follow-ups at 6 and 12 months. The horizontal line in the middle of each box indicates the median, and the top and bottom borders of the box mark the 75th and 25th percentiles, respectively. The whiskers above and below the box mark the 90th and 10th percentiles, respectively.
Thirty-two patients were included in Group B, with a mean age of 9.43 years (range 4–15). The mean values of specific IgE for egg white, and OVM were 9.29kU/l and 7.23kU/l respectively (0.35–100kU/l for both).
Mean size of the wheal after a prick test with egg white and OVM was 9.68mm and 9.69mm, respectively (3–16mm). Only seven out of 32 patients (21.8%) in Group B developed spontaneous tolerance to egg, as opposed to 92.5% in Group A (p<0.0001).
No statistically significant differences were found between groups for: age (p=0.25); age at diagnosis (p=0.48); rate of anaphylactic patients (p=0.28); skin prick test wheal size with egg white (p=0.24) and OVM (p=0.15); and specific IgE for egg white (p=0.95) and OVA (p=0.84).
DiscussionCurrently, when someone is diagnosed with allergy to egg, the only treatment is an elimination diet and therapeutic measures to prepare against accidental intake. However, it is difficult to keep to a strict diet as egg is an ingredient in many foods and is even present latently in some manufactured foods in which the ingredients are not listed. As already pointed out, when it is a matter of a food allergen that is a substance contained within a regular daily diet, as is the case of egg, there is a huge psycho-emotional and economic impact that affects the quality of life of these patients and their family members.8
In 1998, Patriarca et al.7 published desensitisation protocols with different foods (milk, egg, fish and apple), in which five children with egg allergy were included.
Since then, in 2003,9 the same authors published a report on a series of 59 patients who were allergic to various foods. Fifteen of these were allergic to egg, and of these, nine were children between the ages of 3 and 16. Those authors carried out a desensitisation regimen with whole egg, administering pre-treatment with cromoglycate. They prepared a dilution with 10 drops to 100ml water, beginning with one drop of the dilution and making periodic increases until reaching tolerance at 50ml, equivalent to one egg in 139 days. Eight children achieved total tolerance. One patient was withdrawn from the study. They did a follow-up at 18 months with good results, without specifying food.
In 2007, Buchanan et al.12 studied seven non-anaphylactic children who were allergic to egg and aged between 14 months and 7 years and published a tolerance induction protocol with powdered egg white carried out in two phases. In a first rush phase, the authors carried out periodic increases until reaching 300mg. They then went on to a phase in which they maintained the level of 300mg of egg over 24 months. Subsequently they performed a double-blind placebo-controlled challenge test. In this study neither the patients withdrawn from the protocol nor those who achieved partial tolerance are referred to.
Also in 2007, Staden et al.13 carried out a tolerance induction protocol with milk and lyophilised egg in 25 allergic children, of which 11 were allergic to egg. In that study all the patients suffered mild or moderate reactions during the course of the treatment. Those authors, unlike Patriarca et al., did not administer premedication.
Akashi et al.13 reported in the American Academy in 2007 their study in which they included 13 children with egg allergy. Eighty-five percent of the children (11 children) achieved total tolerance and the remaining two tolerated 2 and 7g of egg.
Itoh et al.16 have recently published a study carried out with a rush regimen on six children allergic to egg, aged between 7 and 12. All the patients achieved tolerance and all presented allergic reactions throughout the treatment period.
The range of efficacy in previous studies stands at between 36% and 85%.9,11–20 However the majority of these studies were carried out on young children, which makes it difficult to differentiate the real effect of the treatment from spontaneous tolerance. The average age of our patients was 8.7 years, which is close to the 9-year age that in some studies3 has been identified as being an indicator of poor developmental prognosis. This makes us think that the effect of the treatment is real and not due to the natural evolution of the disease.
In 2010, Vickery et al.17 published their experience with six patients who achieved tolerance to eggs after a SOTI protocol. This method was longer than the previously commented, since the authors repeated specific IgE determinations before each dose increase. However, they observed a significant decrease of egg white and OVM IgE level, as well as an increase of IgG4.
More recently, García Rodríguez et al.18 have developed and successfully tested a rush SOTI protocol in patients with egg allergy. The number of patients in their group of patients was larger than the ones mentioned previously (23 patients) and all but one tolerated a whole cooked egg in just 5 days. They also observed a significant decrease of egg-white specific IgE levels, but not before 6 months after the desensitisation.
In our study, 37 of the 40 patients in Group A (92.5%) achieved tolerance to egg, as opposed to 21.8% in Group B. However, two of the patients included in Group A did not achieve full tolerance. One patient who has bronchial asthma due to sensitivity to fungi suffered various anaphylactic reactions in the clinic during treatment and one had severe reaction at home at a time of stress and rain, resulting in their intake of egg being limited to cooked egg, to which there was good tolerance.
In the literature covered, reactions during treatment occurred in 50–100% of cases.9–20 In our study, 21 of the patients (51.5%) presented symptoms with one dose or more. In 25% the symptoms were mild, such as oropharyngeal pruritis, perioral papulae and mild abdominal pain, which resolved spontaneously or with antihistamines. Thirteen patients presented moderate to severe symptoms, such as moderate abdominal pain, vomiting, urticaria, rhinoconjunctivitis and dyspnoea which needed treatment with corticosteroids and antihistamines in eight patients and epinephrine in another five. Generally, the symptoms occurred in the clinic during the increase in dosage.
Patriarca et al.9 used pre-treatment with cromoglycate in order to minimise undesired effects. Meglio et al.10 carried out a SOTI protocol with cow's milk and administered treatment with cetirizine up until one week following the final dose. Patriarca et al. refer to symptoms during the programme in 51.5% of the patients using cromoglycate, whilst Meglio et al. observed symptoms in 61% of the patients with cetirizine. It is possible that the use of antihistamines during the programme improves the acceptance of the patient once mild symptoms have diminished, such as oral pruritis or perioral outbreaks that could be hidden. In some cases this would mean that either the dose would have to be repeated or the protocol should be continued at a slower pace. Our patients did not systematically undergo pre-treatment with antihistamines. Antihistamine, bronchodilator or anti-inflammatory treatments were only used if required for the treatment of adverse reactions or if indicated by rhinitis comorbidity, atopic dermatitis or asthma to obtain an adequate control whilst avoiding interferences in the assessment of the symptoms.
The majority of the authors used slow regimens with increases made in the clinic and very slowly at home, with control visits for changes of dilution or significant increases. In our SOTI protocol, the food doses were increased weekly in the clinic.
In this study, we did not carry out double-blind placebo-controlled food challenge (DBPCFC), which is the method recommended in research studies. Instead, we used open oral challenge, which is the standard method adopted in clinical paediatric practice.22 We consider that the use of open oral challenge test does not alter the validity of a therapeutic alternative that might present itself to patients who have not spontaneously achieved tolerance to egg, which could change their prognosis and, by so doing, their quality of life.20,22
Previous SOTI studies have shown a decrease in the levels of egg-specific IgE and an increase in the figures for IgG4 once tolerance is achieved.9,10,13,15,19,21 Some studies have found a decrease in the size of the wheal in the cutaneous tests after a minimum period of 6 months from completing the procedure.9,10,15
In our study, both the values of the skin prick tests and the egg-specific IgE and fractions tend to decrease very slowly, remaining similar or greater when tolerance is reached and diminishing latterly. We only identified a more marked decrease, which was statistically significant (p<0.05) in the results of the prick-by-prick tests with egg dilutions once tolerance was achieved. Also, we found statistically significant differences in the total IgG for egg white on completing the procedure.
Although raw egg tolerance has not been tested in all the patients in our study, we consider that cooked egg is enough to eliminate most dietary restrictions, which makes a significant improvement in their quality of life.
There are no previous communications of consecutive SOTI to more than one type of food. Nine of the patients had previously successfully undergone an oral tolerance induction protocol with milk. To our knowledge, this is the first report of consecutive SOTI protocols to more than one type of food.
We consider that our protocol has been safe, effective and of reasonable duration. This type of treatment should be carried out by staff trained in the recognition and treatment of the possible severe reactions which may occur.
Lastly, we consider that the sensitisation rate, expressed using the measurement of specific IgE by the means of skin tests and in vitro methods, is a poor prognostic indicator as far as the duration of the process and the severity of the intercurrent symptoms are concerned. The prick-by-prick skin tests with dilutions of egg have shown themselves to be more sensitive than the total IgE figures. These findings make us think that IgE plays a secondary role as far as tolerance is concerned and might constitute an immunological epiphenomenon, whereas the awareness and modulation of which will become the objective of future investigations.
Ethical disclosureConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.Right to privacy and informed consent. The authors declare that no patient data appears in this article.Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestAuthors disclose any financial relationship which can cause a conflict of interest regarding this article.
This project has not been published in any other journal. However, preliminary results and shorter series have been presented at:
- •
XXVI Congreso Nacional de la Sociedad Española de Alergología e Inmunología Clínica. Bilbao, Spain, November 6–8, 2008.
- •
XXXIII Congreso de la Sociedad Española de Inmunología Clínica y Alergología Pediátrica. Palma de Mallorca, Spain, May 14–16, 2009.
- •
XXVIII Congress of the European Academy of Allergology and Clinical Immunology. Warsaw, Poland, June 6–10, 2009.
- •
1st International Congress Southern European Allergy Societies. Florence, Italy, March 18–20, 2010.
- •
XXVII Congreso Nacional de la Sociedad Española de Alergología e Inmunología Clínica. Madrid, Spain, November 10–13, 2010.
It was awarded the 2nd prize for the best communications at the XXXIII Congreso de la Sociedad Española de Inmunología Clínica y Alergología Pediátrica. Palma de Mallorca, Spain, May, 2009 for the abstract entitled “Inducción de Tolerancia oral a huevo en pacientes con alergia persistente”, by V. Fuentes Aparicio, E. Alonso Lebrero, L. Gacías Pedrós, A. Vásquez Bautista, and L. Zapatero Remón.
The authors wish to thank Dra. M.I. Martínez Molero, head department (retired) for her encouragement and collaboration in the development of this new therapeutic approach, as well as the nurses Teresa Pérez, Patricia González, Francisca López and the rest of the nursing staff for their excellent work.