X-linked agammaglobulinaemia (XLA) is a genetic disorder affecting B cell maturation, which is characterised by a low number of B cells, agammaglobulinaemia and increased susceptibility to a variety of bacterial infections. This study was performed to assess T cell subpopulations in a group of children with XLA in association with chronic respiratory disease (CRD).
MethodsNumbers of T cell subpopulations (CD3+, CD4+, CD8+, CD3+DR+, naïve, memory, recent thymic emigrants (RTE), regulatory T cells, follicular T helpers) were measured by eight-colour flow cytometry in 22 XLA patients and 50 controls. BAFF level was measured by ELISA.
ResultsXLA patients with CRD had a significantly lower percentage of RTE numbers and Tregs, while significantly higher absolute counts of lymphocytes, CD3+, CD8+, CD3+DR+ and CD4+CD45RO+ T cells were detected as compared with healthy controls. In patients with XLA without CRD, the number of follicular T helper cells was altered significantly (percentage and absolute), as compared with healthy controls. Additionally, they had significantly higher counts (percentage and absolute) of CD4+CD45RA+ cells and lower percentage of CD4+CD45RO+ cells in comparison with healthy controls.
ConclusionsOur study affords new information concerning CRD and T cell subsets that differentiate or are maintained in the absence of B cells in children with XLA. T cell's homeostasis depends on the presence of chronic respiratory disease that may be caused by the delay in diagnosis.
X-linked agammaglobulinaemia (XLA) is a primary immunodeficiency, which is characterised by a reduction in all classes of serum immunoglobulins and absence of B cells (CD19+ cells) due to the mutation in Bruton's tyrosine kinase (Btk). Btk blocks the maturation of B cells at the pro-B-pre-B1 stage in bone marrow, generally leading to a very low number of peripheral B cells (usually less than 1%) and clinically is presented by recurrent bacterial infections in the affected males in the first two years of life [1–4].
Despite major advances over the last 20 years in the molecular characterisation of primary immunodeficiency diseases (PIDs), many patients still go undiagnosed or are diagnosed late, with adverse clinical consequences [5]. It has been shown that delayed diagnosis of patients with X-linked agammaglobulinaemia leads to an increased risk of chronic respiratory disease. About 40% patients who are not diagnosed until the age of 10 years have the chance of developing chronic lung disease, while if the diagnosis is made after 15 years, the risk could be twice as high [6]. Well-timed diagnosis and regular treatment of agammaglobulinaemia with intravenous immunoglobulin (IVIG) significantly reduced the incidence of pneumonia and hospital admission [7].
Chronic respiratory disease (CRD) is a group of chronic diseases affecting the airways and other structures of the lungs. Respiratory infections are the most frequent manifestations appearing at the diagnosis of XLA and during the disease progression [6–11].
Despite a number of studies in immunological abnormalities of adult cases with XLA, there is no paediatric study describing T cell subpopulation abnormalities. This study was designed to assess T cell homeostasis in children with XLA and/or with CRD. Moreover, high concentration of BAFF was described in many patients with immunodeficiency [12,13] and congenital agammaglobulinaemia [12,20,21]. Therefore, we investigated the role of chronic respiratory disease in BAFF concentration in XLA patients.
Patients and methodsEthics statementWritten informed consent was obtained from all the parents of patients and healthy children before sampling. The study was approved by the Ethical Committee of the Belarusian Research Centre for Paediatric Oncology, Haematology and Immunology (BRCPOHI).
Patients and controlsPeripheral blood samples were taken from 22 male patients with XLA. Eight patients were diagnosed and regularly seen in BRCPOHI, Minsk, Belarus, while 14 patients were from the Russian Children's Clinical Hospital, Moscow, Russia. All patients were divided into two groups according to the presence of chronic respiratory disease (CRD): chronic lung disease (CLD) and/or chronic sinusitis. Chronic lung diseases included chronic bronchitis [evidence of productive cough, dyspnoea and wheezing with computed tomography (CT) scan alterations], bronchiectasis [evidence of daily cough and purulent sputum production associated with abnormal chest or CT scan radiographs]. Chronic sinusitis was diagnosed when two of three major signs were evident: rhinorrhoea, postnasal drip, and coughing for at least three months.
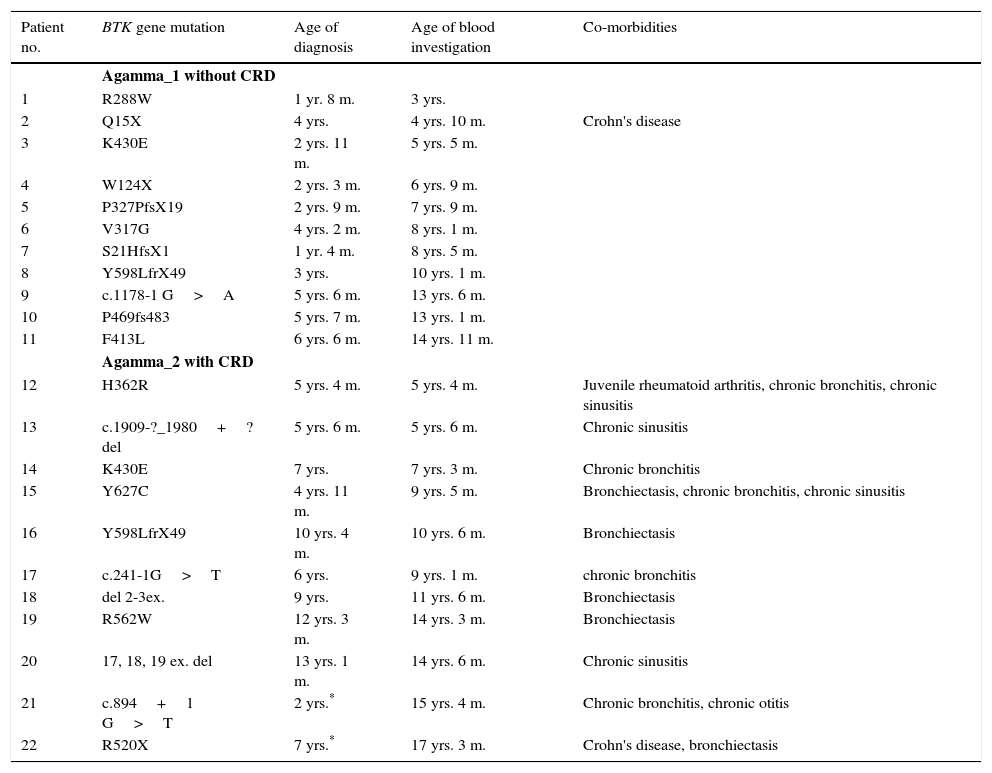
The first group (Agamma_1) included 11 patients without CRD (age range of 3–15 years, median eight years); the second group (Agamma_2) included 11 patients with CRD (age range of five years and five months to 17 years and five months, median 10.6 years) (Table 1). All 22 patients with XLA had mutation in Btk gene (Table 1).
Clinical and genetic characteristic of XLA patients. Mutations of patients, ages at diagnosis, ages of blood investigation and co-morbidities.
| Patient no. | BTK gene mutation | Age of diagnosis | Age of blood investigation | Co-morbidities |
|---|---|---|---|---|
| Agamma_1 without CRD | ||||
| 1 | R288W | 1 yr. 8 m. | 3 yrs. | |
| 2 | Q15X | 4 yrs. | 4 yrs. 10 m. | Crohn's disease |
| 3 | K430E | 2 yrs. 11 m. | 5 yrs. 5 m. | |
| 4 | W124X | 2 yrs. 3 m. | 6 yrs. 9 m. | |
| 5 | P327PfsX19 | 2 yrs. 9 m. | 7 yrs. 9 m. | |
| 6 | V317G | 4 yrs. 2 m. | 8 yrs. 1 m. | |
| 7 | S21HfsX1 | 1 yr. 4 m. | 8 yrs. 5 m. | |
| 8 | Y598LfrX49 | 3 yrs. | 10 yrs. 1 m. | |
| 9 | c.1178-1 G>A | 5 yrs. 6 m. | 13 yrs. 6 m. | |
| 10 | P469fs483 | 5 yrs. 7 m. | 13 yrs. 1 m. | |
| 11 | F413L | 6 yrs. 6 m. | 14 yrs. 11 m. | |
| Agamma_2 with CRD | ||||
| 12 | H362R | 5 yrs. 4 m. | 5 yrs. 4 m. | Juvenile rheumatoid arthritis, chronic bronchitis, chronic sinusitis |
| 13 | c.1909-?_1980+?del | 5 yrs. 6 m. | 5 yrs. 6 m. | Chronic sinusitis |
| 14 | K430E | 7 yrs. | 7 yrs. 3 m. | Chronic bronchitis |
| 15 | Y627C | 4 yrs. 11 m. | 9 yrs. 5 m. | Bronchiectasis, chronic bronchitis, chronic sinusitis |
| 16 | Y598LfrX49 | 10 yrs. 4 m. | 10 yrs. 6 m. | Bronchiectasis |
| 17 | c.241-1G>T | 6 yrs. | 9 yrs. 1 m. | chronic bronchitis |
| 18 | del 2-3ex. | 9 yrs. | 11 yrs. 6 m. | Bronchiectasis |
| 19 | R562W | 12 yrs. 3 m. | 14 yrs. 3 m. | Bronchiectasis |
| 20 | 17, 18, 19 ex. del | 13 yrs. 1 m. | 14 yrs. 6 m. | Chronic sinusitis |
| 21 | c.894+1 G>T | 2 yrs.* | 15 yrs. 4 m. | Chronic bronchitis, chronic otitis |
| 22 | R520X | 7 yrs.* | 17 yrs. 3 m. | Crohn's disease, bronchiectasis |
The healthy control (HC) group consisted of 50 children with the age range of three to 18 years (median age 10.7 years). Diseased controls were recruited from the pulmonology units of Children's Clinics, Minsk, in order to control the effect of chronic infection on the T cell subpopulations. There were 10 patients (six boys, four girls) (CLD – chronic lung disease control group) with bronchiectasis (n=7) and chronic bronchitis (n=3) (age range of 5–17 years, median 9.5 years) without agammaglobulinaemia. The patients were selected by pulmonologists, based on CT confirmed bronchiectasis and chronic bronchitis without humoral immunodeficiency. Children with asthma, tuberculosis and cystic fibrosis were excluded from the study.
All patients and children from the control groups were free of infection (C-reactive protein level was normal), when T cell analysis was performed.
Phenotyping of peripheral blood lymphocytesPeripheral blood (PB) samples were analysed in the Laboratory of Immunology, BRCPOHI, Belarus. The analysis of PB lymphocyte subpopulations was performed eight-colour flow cytometry (Navios, Beckman-Coulter, Brea, USA), using 100μl of whole blood stained with the following monoclonal antibodies (MoAbs) at optimal concentrations against CD45, CD3, CD4, CD8, CD16/CD56, HLA-DR, CD19, CD45RA, CD45RO, CD31, CD127, CD25, PD1 (all obtained from Beckman-Coulter, Marseille, USA), CXCR5 (R&D, Minneapolis, USA) and incubated in the dark for 10min at room temperature. The MoAbs were conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), phycoerythrin-Texas-Red-Tandem (ECD), phycoerythrin-cyanin-5 (PC-5), phycoerythrin-cyanin-7 (PC-7), allophycocyanin (APC). The following CD3+ and CD4+ T cell subpopulations were analysed: naive (CD3+CD4+/CD8+CD45RA+), memory (CD3+CD4+/CD8+CD45RO+), recent thymic emigrants (CD4+CD45RA+CD31+), putative follicular T cells (CD4+CXCR5+PD1+), putative follicular memory T cells (CD4+CXCR5+CD45RO+) and Tregs (CD4+CD25+CD127low). An automated lyse/no-wash procedure with a fixation step followed using a TQ-PrepTM Workstation (Beckman Coulter, Brea, USA) with the ImmunoPrep reagent. Data were analysed using CXP analysis software, Kaluza Flow Cytometry Analysis v1.2 (Beckman Coulter, City).
Btk genotypingThe Btk was sequenced by sequencing coding region, exon-intron boundaries, and known pathogenic intronic sequence variations. Sequence analysis was performed by «Genetic Analyzer ABI 3130» (Hitachi, Japan) and then aligning to reference sequence (ENST00000308731:c.). Mutations in patient's no. 4, 7, 10, 15, 17, 21, 22 were detected in the Russian Federation.
Enzyme-linked immunosorbent assay (ELISA)BAFF concentrations in serum samples were measured using commercially available ELISA kits (Human BAFF/BlyS/TNFSF13B Quantikine ELISA Kit, R&D Systems, Inc., Minneapolis, MN, USA), following the manufacturer's instructions.
Statistical analysisStatistical analysis was performed using GraphPad Prism version 5.0 (GraphPad Software Inc., CA, USA). The obtained data were analysed using non-parametric one-way analysis of variance (ANOVA) Kruskal–Wallis with Dunn's multiple comparison test as a post hoc test. Mann–Whitney U test was used for detection between two variables. Spearman's coefficient was used to determine the significance of the correlation between two variables. The data are presented as medians and whiskers (min–max). The differences between the groups were considered significant with p<0.05.
ResultsCharacteristics of patients with agammaglobulinaemiaAt the time of diagnosis, 11 out of 22 (50%) patients had CRD (Agamma_2) (Table 1). The median age at diagnosis of these patients was higher and significantly different as compared with that of the remaining 11 patients without CRD (Agamma_1) [7.2 years (range: 5.3–13.1 years) vs. 3.6 years (range: 1.7–6.5 years) p<0.05].
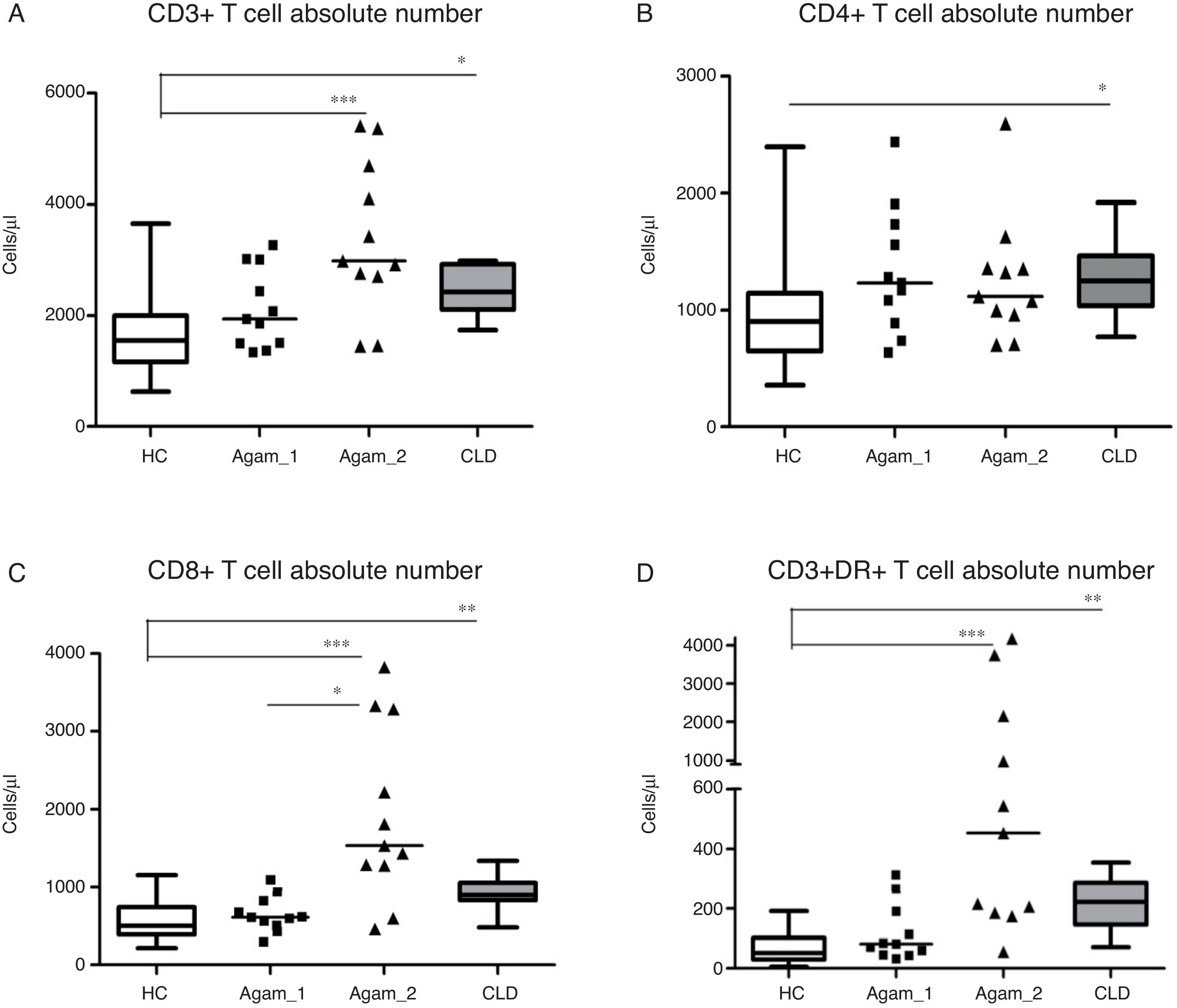
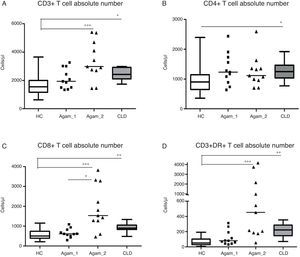
Comparison of T cell subpopulation absolute counts in patients with agammaglobulinaemiaInvestigation of the main lymphocyte subsets in patients and control groups revealed that XLA patients with chronic infections (Agamma_2) and children with CLD had significantly higher lymphocyte numbers as compared with HC (data not shown). CD3+ (p<0.001, p<0.05), CD8+ (p<0.001, p<0.01), CD3+DR+ (p<0.001, p<0.01) cells counts in Agamma_2 and CLD cases were also significantly higher than HC. CD8+ cells counts in Agamma_2 patients were significantly higher than those with Agamma_1 (p<0.05) (Fig. 1A–C). There was no significant difference in CD4+ T cells between the patients and control groups except significant increase in CLD children vs. HC (Fig. 1B). NK cells were significantly lower in Agamma_1 patients as compared with CLD. No other significant difference was observed (data not shown).
(A–D) CD3+, CD4+, CD8+ and CD3+DR+ T cell counts (Cells/μl) in patients with agammaglobulinaemia and control groups. HC – healthy controls, Agamma_1 – XLA patients without chronic lung disease, Agamma_2 – XLA patients with chronic lung disease, CLD – children with chronic lung disease without XLA.
CD3+ cell number was found to positively correlate with CD8+ T cells (r=0.65, p=0.029) in Agamma_1 and (r=0.87, p=0.0009) in Agamma_2. Similar relationships were observed in HC (r=0.87, p<0.001). In contrast to all previous groups, no association was found between the number of CD3+ T cells and CD8+ T cells (r=0.42, p=0.23) in children with CLD. Therefore, most of the effects on the T cell compartment (absolute number) are comparable in agammaglobulinaemia patients with CRD and the CLD control group.
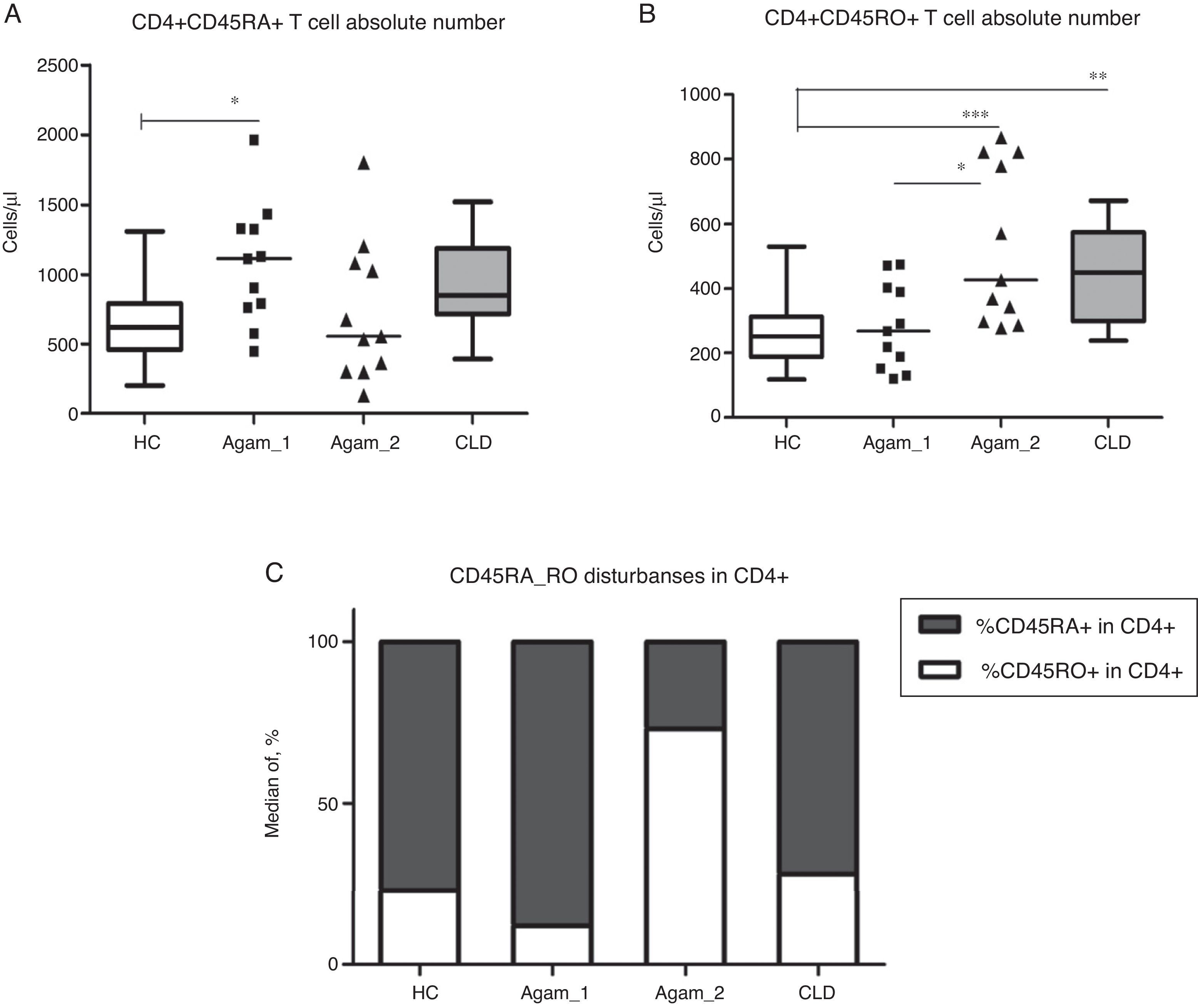
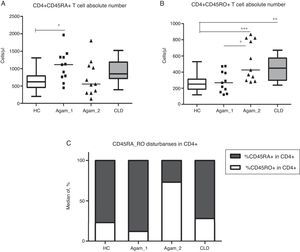
CD4+ T cell subpopulation absolute countsAs described earlier, patients with XLA had reduced absolute numbers of memory CD4+CD45RO+ T cells [3,14,15]. Among the groups of patients, significantly higher numbers of CD4+CD45RO+ cells were observed in Agamma_2, as compared to the HC and Agamma_1 patients (p<0.001, p<0.05). The same observation was noticed in children with CLD (p<0.05) once compared to HC (Fig. 2B). Absolute numbers of naive CD4+CD45RA+ T cells were significantly higher in patients in Agamma_1 as compared to HC (p<0.05) (Fig. 2A). Therefore, the percentage distribution of CD45RA and CD45RO among CD4+ T cells was analysed. CD45RA+ proportion among CD4+ T cells was significantly higher in Agamma_1, as compared to HC (p<0.05) and significantly lower in Agamma_2 and children with CLD, as compared to Agamma_1 (p<0.001, p<0.01, respectively). A similar but inverted situation was found in CD45RO+ among CD4+ T cells in patients with Agamma_2 (Fig. 2C). No additional significant difference was found between XLA patients and controls.
(A) CD4+CD45RA+ T cell counts (Cells/μl) in patients with agammaglobulinaemia and control groups. (B) CD4+CD45RO+ T cell counts (Cells/μl) in patients with agammaglobulinaemia and control groups. (C) Percent distribution of CD45RA among CD4+ T cells in patients with agammaglobulinaemia and control groups. HC – healthy controls, Agamma_1 – XLA patients without chronic lung disease, Agamma_2 – XLA patients with chronic lung disease, CLD – children with chronic lung disease without XLA.
The domination of T cells with phenotype CD4+CD45RO+ in XLA patients with CRD may be caused by constant stimulation of the immune system with chronic infection.
CD8+ T cell subpopulation absolute countsNo significant difference in the analysis of CD8+CD45RA+ and CD8+CD45RO+ T cells was found in patients with XLA without CRD (Agamma_1). However, Agamma_2 and children with CLD had significantly higher absolute numbers of CD8+CD45RA+ T cells as compared to HC (p<0.001, p<0.001, respectively) and higher absolute counts CD8+CD45RO+ T cells as compared to HC (p<0.001, p<0.01 respectively), and Agamma_2 as compared to Agamma_1 (p<0.05) (data not shown). All these differences are due to the increased CD8+ T cells’ numbers in Agamma_2 and children with CLD.
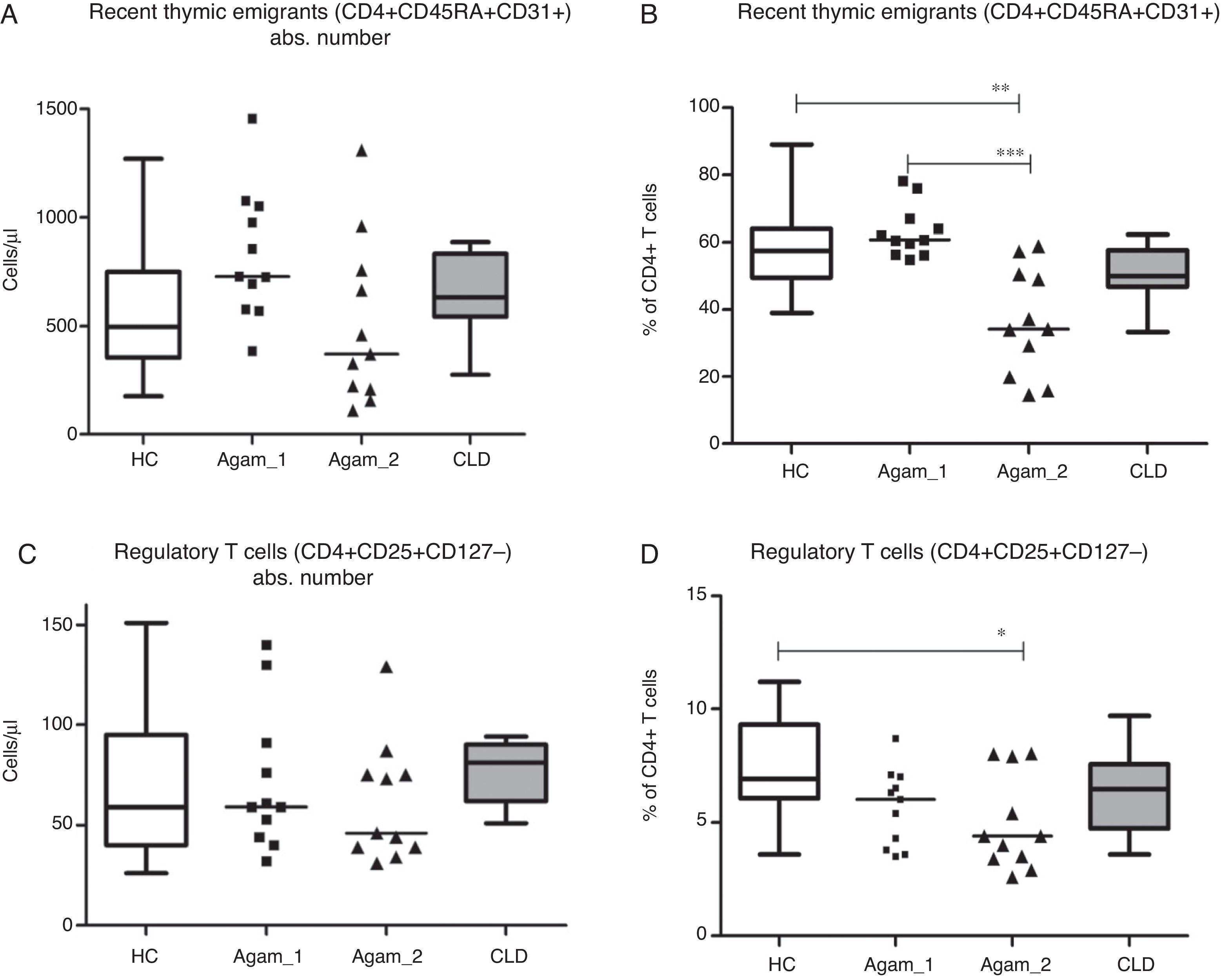
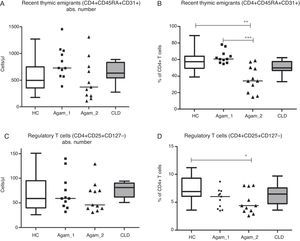
Recent thymic emigrants (RTE), Tregs and putative follicular T cellsAbsolute numbers of recent thymic emigrants (RTE) and regulatory T cells (Treg) in both groups of XLA patients were comparable to healthy controls and CLD controls (p>0.05). The percentage of RTE was significantly lower in Agamma_2 patients as compared to healthy controls and Agamma_1 patients (p<0.01, p<0.001) (Fig. 3A) and the percentage of Treg was significantly lower in Agamma_2 patients as compared to healthy controls (p<0.05) (Fig. 3B).
(A and B) Absolute counts and the percentage of recent thymic emigrants in patients with agammaglobulinaemia and control groups. (C and D) Absolute counts and the percentage of regulatory T cells in patients with agammaglobulinaemia and control groups. HC – healthy controls, Agamma_1 – XLA patients without chronic lung disease, Agamma_2 – XLA patients with chronic lung disease, CLD – children with chronic lung disease without XLA.
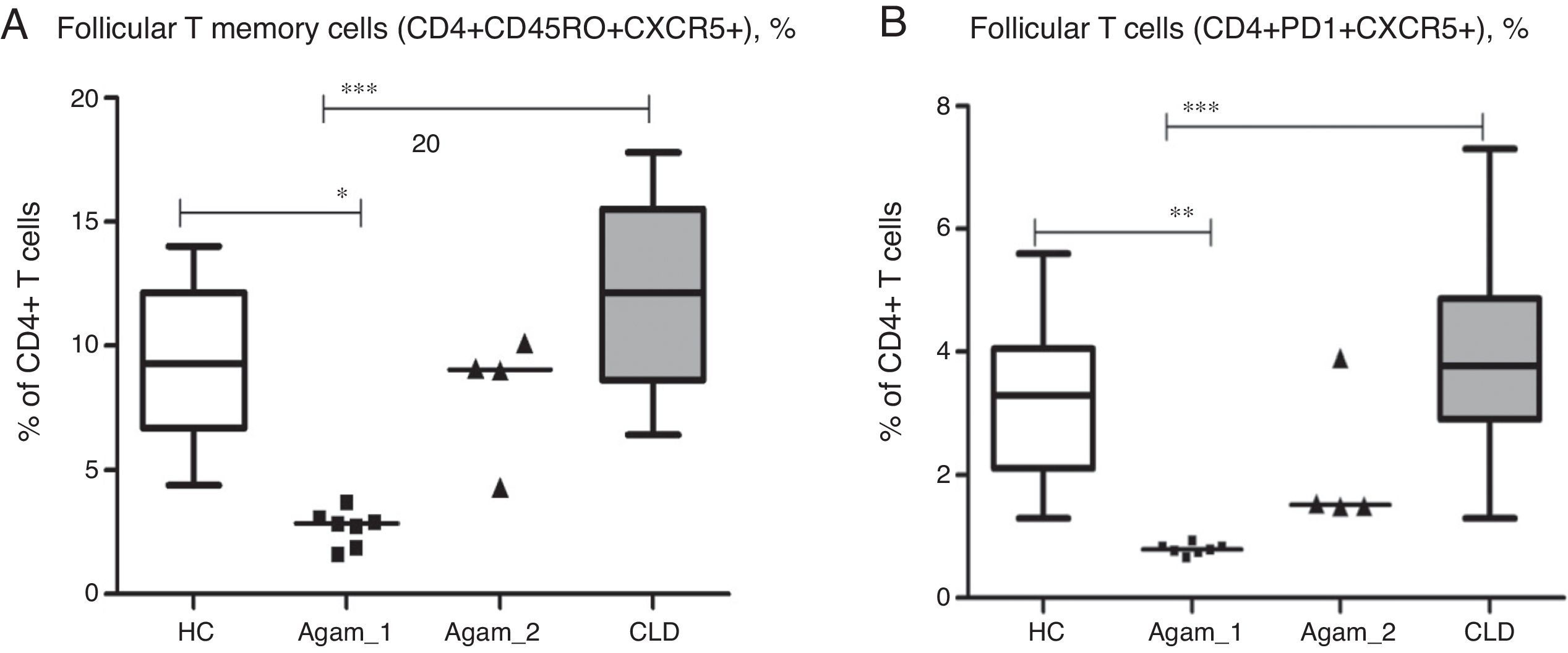
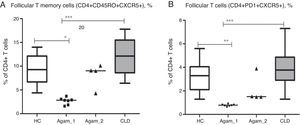
Additionally, for 11 XLA patients (7 – Agamma_1, 4 – Agamma_2) the number of follicular T helper cells was investigated. The numbers of putative follicular memory T cells (CD4+CD45RO+CXCR5+) were significantly lower in patients with Agamma_1 (percentage and absolute) as compared to HC and children with CLD (% p<0.05, p<0.001; abs. p<0.05, p<0.001) (Fig. 4A). Percentages and absolute numbers of follicular helper T cells – with phenotype (CD4+CXCR5+PD1+) were significantly lower in patients with Agamma_1 as compared to those of HC and children with CLD (% p<0.01, p<0.001; abs. p<0.01, p<0.001) (Fig. 4B).
(A) The percentage of putative follicular memory T cells (CD4+CD45RO+CXCR5+) in patients with agammaglobulinaemia and control groups. (B) The percentage of putative follicular T cells (CD4+CD45RO+CXCR5+) in patients with agammaglobulinaemia and control groups. HC – healthy controls, Agamma_1 – XLA patients without chronic lung disease, Agamma_2 – XLA patients with chronic lung disease, CLD – children with chronic lung disease without XLA.
The results confirm that normal T helper request B cells for normal differentiation or survival [13–15]. No significant difference between Agamma_2 patients and controls was found when analysing percentage and absolute counts of follicular T cells as assessed by different markers. Normal differentiation of TFH could result from repeated infections, as well as the higher numbers of primed CD4+CD45RO+ T cells in this group of patients with Agamma_2. The small sample size affords no possibility to investigate such a hypothesis.
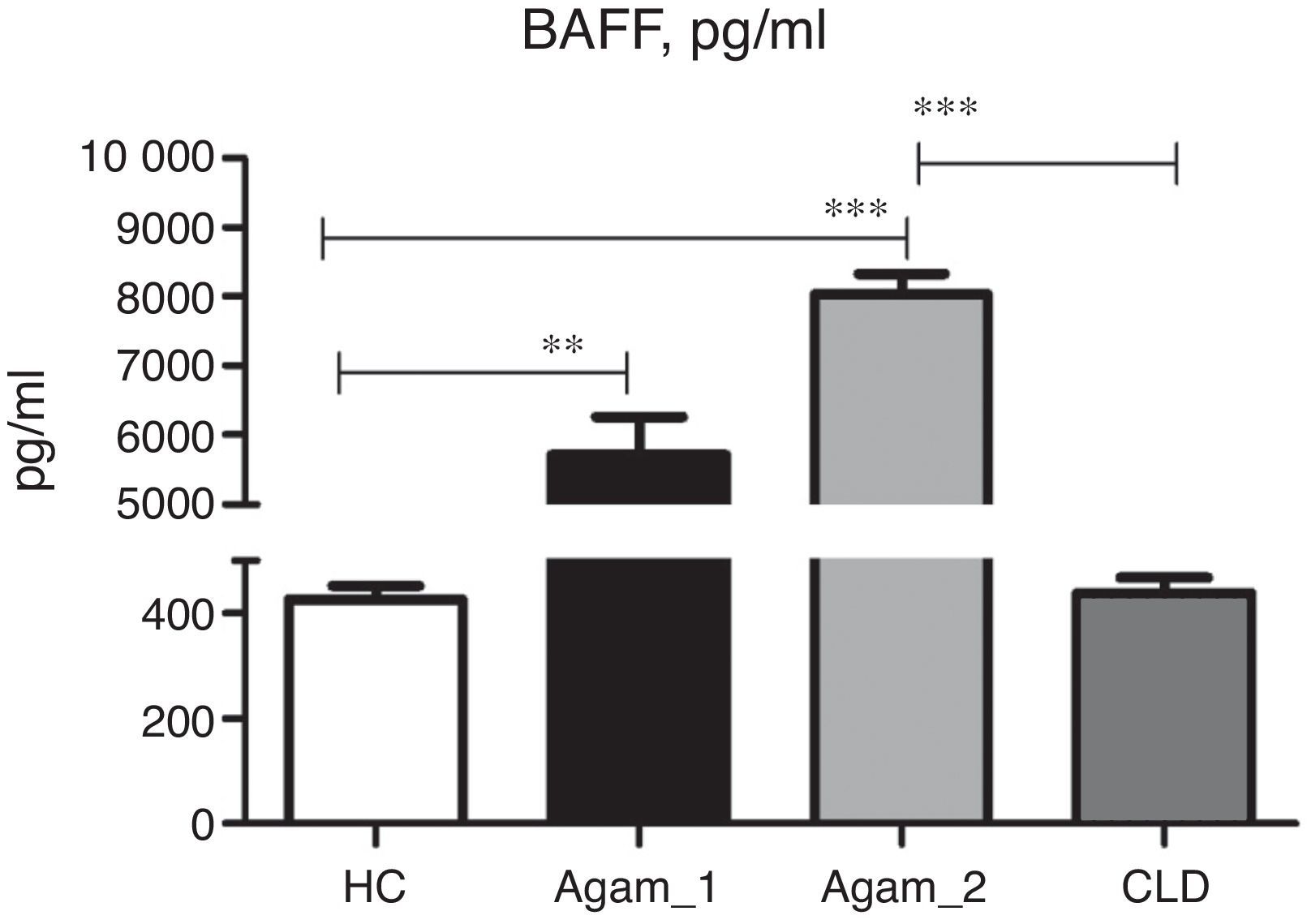
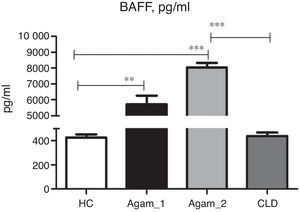
BAFF concentration in XLA patientsSoluble BAFF was detected in all groups of patients and controls. In CLD patients, the BAFF serum concentration was very similar to HC (Fig. 5). Very high BAFF concentrations were detected in samples of Agamma_1 and Agamma_2 patients as compared to HC (p<0.01, p<0.001). XLA patients with chronic lung disease had significantly higher BAFF level in comparison with CLD children (p<0.001) as determined by ANOVA.
Mann–Whitney analysis between the data of two groups of XLA patients showed a significantly higher level in Agamma_2 (p=0.003).
DiscussionThe main idea of this work was to test the effect of chronic infection on T lymphocytes and BAFF in children with the absence of B-lymphocytes. Our results show, in agreement with these studies, that chronic infection additionally contributes to an increase in BAFF level in patients with XLA.
In most cases, XLA is successfully diagnosed at three years old and adequate immunoglobulin therapy is administered. However, due to a lack of awareness by physicians, there is a risk of late diagnosis after 5–10 years when the boys have a lot of infectious complications, in particular, permanent damage to the lung tissue – bronchiectasis [5,6]. In this study, we evaluated that XLA patients with chronic lung disease have a significantly lower percentage of RTE numbers and Tregs and have significantly higher absolute counts of lymphocyte numbers, CD3+, CD8+, CD3+DR+ and CD4+CD45RO+ T cells as compared to healthy controls. Absence of chronic infections in XLA patients significantly altered the percentages and absolute numbers of follicular T helper cells, together with CD4+CD45RO+ cells as compared to healthy controls. Our findings confirm a relationship between chronic infection and changes in the immunity of patients with inborn absence of B cells.
Currently, there are some contrasting data about the effect of B cells on T cell numbers and phenotypic abnormalities [14–16]. A previous study, including 16 patients with XLA and nine patients with common variable immunodeficiency (CVID), who had less than 2% B-lymphocytes in the peripheral blood, showed that the compartment of CD4+ T-memory cells was significantly reduced in patients with XLA and CVID as compared to age-matched controls. That study revealed a significant B-lymphocytes’ contribution to the differentiation of T-helper cells [2]. T-helper 17 percentage was reduced in patients with agammaglobulinaemia, in spite of the absence of clinical hypersensitivity to Candida albicans in this group of patients [17]. The deepest multi-parameter T-lymphocytes surface markers analysis was further described [18]. In patients with XLA, significantly reduced numbers of absolute CD4+ effector memory T-cells (CD4+CD45RA−CCR7−) and follicular helper T-cells (CD4+CD45RO+CXCR5+) were detected; although a significantly increasing trend of naive CD4+ T-cells and thymic emigrants was identified in patients as compared to controls [18].
However, the presence of congenital X-linked agammaglobulinaemia has an additional effect on depletion of RTE pool and expansion of CD4+CD45RO+. Abnormalities in the RTE percentages may be caused by shifts in other T cell subsets, dominating CD4+CD45RO+ T cells in the blood of patients with agammaglobulinaemia and CRD. We observed the predominance of memory T cells subsets in both CD4+ and CD8+ T cells’ subsets in XLA patients with chronic lung disease possibly as a result of constant presence of infectious agents. It has been suggested that chronic infection is the reason for CD45RO+ dominance.
XLA is a primary immunodeficiency disorder with an increased risk of autoimmunity of up to 15% [19]. The first hypothesis was that the constant stimulation of the immune system might be the cause of autoimmunity. However, in our study, this hypothesis was not confirmed. We observed auto inflammatory disorders in the anamnesis of three patients among 22 XLA patients (13.6%). In Agamma_2 group n=2, one patient had juvenile rheumatoid arthritis (JRA) (n=1, Pt no. 17) and one – Crohn's disease (CD) (Pt no. 27), in Agamma_1 n=1: Crohn's disease (Pt no. 3). The onset of auto inflammation was observed at the ages of 3, 17 and 5 years. Pt_17 and Pt_27 had chronic infections at the moment of blood investigation (Table 1, 3), but Pt_17 was diagnosed with JRA at the age of three years before the appearance of chronic lung infections.
Lack of B lymphocytes affects some subset of T lymphocytes (T follicular helper cells), but in children with chronic infections we found no abnormalities in the formation of this population, despite the absence of B lymphocytes. For detection of circulating “bona fide” memory T cells (TFH), we used CD4+CD45RO+CXCR5+ and CD4+PD1+CXCR5+ phenotype. No significant differences between XLA patients with chronic lung disease and controls were discovered when analysing percentage and absolute counts of follicular T cells whatever the markers used for detection were. A role of chronic infection in the generation/differentiation of TFH can thus be suspected when B cells are lacking.
High concentration of BAFF was described in many patients with immunodeficiency [12,13], and congenital agammaglobulinaemia [12,20,21]. The B cell-activating factor of the TNF family (BAFF) binds with different affinities to three different TNFR-like proteins expressed by B cells termed BAFF-R, TACI, and BCMA. Binding to BAFF-R activates NFκB signalling pathways, resulting in the expression of a series of genes that are essential for B cell survival. It was shown that soluble BAFF concentrations might change during ageing, in response to infection and autoimmunity [12]. However, the mechanism of immune dysregulation during the increase of BAFF in B cell absence remains unclear [12]. Very high BAFF concentrations were detected in samples of Btk patients. These data suggest that the number of B cells, and thus the number of available BAFF binding sites is one of the most critical parameters in regulating the steady-state concentrations of soluble BAFF. Since the soluble BAFF concentration may change during ageing in response to infection [12], we compared the BAFF concentration in samples of XLA patients with different infectious status.
In conclusion, our study affords new information concerning CLD and T cell subsets that differentiate or are maintained in the absence of B cells in children with XLA.
Author contributionsS.Sh. performed the T cells phenotyping, I.G. and A.M. performed genetic investigation, O.P. and I.K. took care for patients from Russia and prepared blood samples, S.Sh., I.K., O.A. designed study, S.Sh. wrote the paper. This work was supported by grants from the Belarusian Ministry of Health.
Conflict of interestThe authors have no financial conflicts of interest to disclose.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
We are most grateful to the patients and controls who generously donated blood samples. We are also grateful to Doctor Anne Durandy and Doctor Nima Rezaei for critical reading of our manuscript.