Sinomenine (SIN), an alkaloid isolated from the root of Sinomenium acutum which has a variety of pharmacological effects, including anti-inflammation, immunosuppression and anti-angiogenesis. The present study aimed to evaluate the effects of SIN on airway remodelling, epithelial apoptosis, and T Helper (Th)-2 derived cytokine levels in a murine model of chronic asthma.
MethodsTwenty-two BALB/c mice were divided into four groups; I (control), II (placebo), III, IV. Mice in groups III and IV received the SIN (100mg/kg), and dexamethasone (1mg/kg) respectively. Epithelium thickness, sub-epithelial smooth muscle thickness, number of mast and goblet cells of samples isolated from the lung were measured. Immunohistochemical scorings of the lung tissue for matrix metalloproteinase-9 (MMP-9), vascular endothelial growth factor (VEG-F), transforming growth factor-beta (TGF-β), terminal deoxynucleotidyl transferase-mediated dUTP nick endlabeling (TUNEL) and cysteine-dependent aspartate-specific proteases (caspase)-3 were determined. IL-4, IL-5, IL-13, Nitric oxide in bronchoalveolar lavage fluid (BALF) and ovalbumin-specific immunoglobulin (Ig) E in serum were quantified by standard ELISA protocols.
ResultsThe dose of 100mg/kg SIN treatment provided beneficial effects on all of the histopathological findings of airway remodelling compared to placebo (p<0.05). All cytokine levels in BALF and serum and immunohistochemical scores were significantly lower in 100mg/kg SIN treated group compared to the placebo (p<0.05).
ConclusionsThese findings suggested that the dose of 100mg/kg SIN improved all histopathological changes of airway remodelling and its beneficial effects might be related to modulating Th-2 derived cytokines and the inhibition of apoptosis of airway epithelial cells.
Bronchial asthma is associated with chronic airway inflammation and progressive airway remodelling.1 The hallmarks of the structural changes in the airways include eosinophilic airway inflammation, airway smooth muscle and goblet cell hyperplasia, collagen deposition, angiogenesis and apoptosis.2 Current anti-inflammatory treatment of asthma is predominately based on the use of inhaled corticosteroids. Although these drugs are highly effective in preventing life-threatening consequences of asthma,3 their effect is limited in modulating airway remodelling and they have several systemic and local side effects when used at high doses for a long time.4 Research for the use of alternative and complementary treatments that reverse airway remodelling and have fewer side effects is continuously increasing.
Sinomenine (SIN) is an alkaloid that is isolated from the root and stem of the climbing plant Sinomenium acutum and demonstrates anti-inflammatory, immunosuppressive, anti-arrhythmic, analgesic and anti-rheumatic effects.5–9 It has been successfully used for centuries in the treatment of rheumatism.10 Only few studies in the literature have investigated the mechanism of action and efficacy of SIN on allergic inflammation.11–13
In our study, we firstly investigated the effects of SIN on airway remodelling, airway epithelial cell apoptosis and TH-2 immune responses in a murine model of chronic asthma in comparison with the conventional dexamethasone treatment.
Materials and methodsAnimals, experimental protocol and study drugsA total of twenty-two, conventionally raised, 6–8-week-old male BALB/c mice weighing 18–20g were used in the study. The animals were fed a commercial diet ad libitum and housed in hygienic macrolane cages in air-conditioned rooms on a 12-h light/dark cycle. All experimental procedures complied with the requirements of the Animal Care and Ethics Committee of the Dokuz Eylul University.
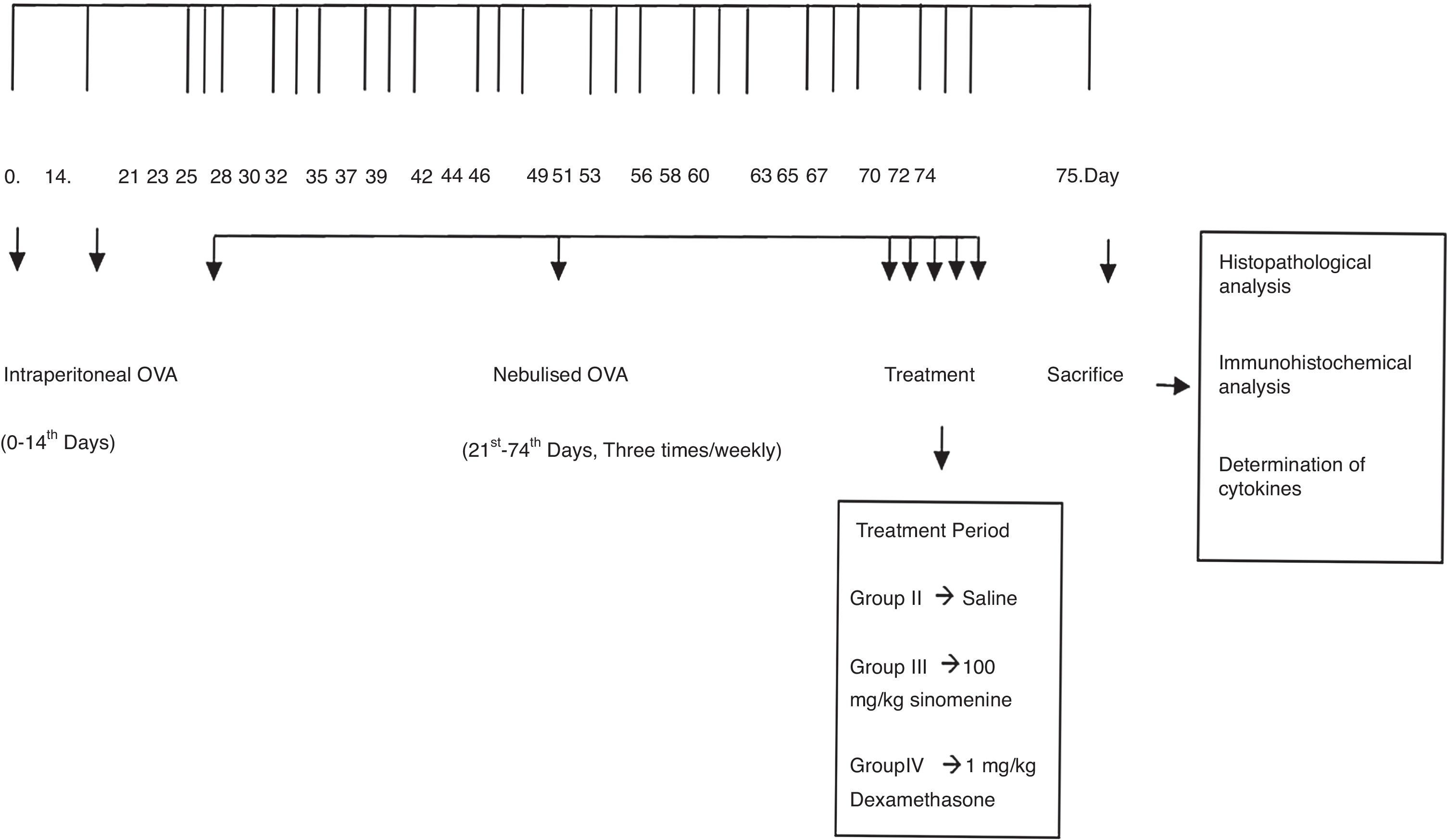
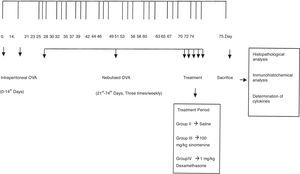
Mice were divided into four groups: (I) Control (n=5), (II) Asthma-untreated (placebo) (n=5), (III) Asthma-100mg/kg SIN-treated (n=6), (IV) Asthma-1mg/kg dexamethasone treated (n=6). Mice in study groups except for the control group were sensitised on days 0 and 14 by an intraperitoneal (i.p) injection of 10μg/0.1ml chicken egg albumin (ovalbumin, grade V, ≥98% pure, Sigma, St. Lois, MO, USA) with alum as an adjuvant as described by Temelkovski et al.14 Mice in groups II, III and IV were challenged with an aerosol of 5ml, 2.5% ovalbumin in saline for 30min/day for three days of the week for eight weeks beginning from the 21st day of the study (Fig. 1). The mice in the control group received normal saline with alum intraperitoneally on days 0 and 14 of the experiment and aerosolised saline without alum for 30min per day on three days of the week for eight weeks beginning from the 21st day of the study. Exposures were carried out in a whole-body inhalation exposure system in a plexiglass chamber with 40×60×120 diameters designed for placement of cages. Temperature and relative humidity were maintained at 20–25°C and 40–60%, respectively. A solution of 2.5% ovalbumin in normal saline was aerosolised by delivery of compressed air to a sidestream jet nebuliser with a flow rate of 6L/min (Medi-cair, UK) and injected into a chamber. The aerosol generated by this nebuliser comprised >80% particles with a diameter of <4μm. Particle concentration was maintained in the range of 10–20mg/mm3 in the chamber.15
During the last five days of the challenge period, group II received saline, group III received SIN (Sigma Aldrich, St. Louis, MO, USA) at dose of 100mg/kg, group IV received dexamethasone (Dekort; Deva Holding AS, Istanbul, Turkey) at dose of 1mg/kg by orogastric tube, once a day. Animals were sacrificed by an overdose of ketamine hydrochloride (200mg/kg) 24h after the last drug administration.
Histopathological analysisTwo investigators who were blinded to the treatment groups interpreted the histopathology. Tissue specimens were obtained from the mid-zone of the left lung of mice. Samples were fixed in buffered 10% formalin and embedded in paraffin wax. Five-micron-thick serial sections were obtained and the first 10 samples were stained with haematoxylin and eosin (H&E). General tissue features of these samples were examined and the thicknesses of epithelium and sub-epithelial smooth muscle layers of the medium and small airways were measured. In order to evaluate the thicknesses of epithelium and sub-epithelial smooth muscle layers, measurements were performed from four points of each airway. Considering that each section contained approximately two to three airways, around 20 or more airways were evaluated for each mouse. Photomicrographs were taken by Olympus DP71 camera (Japan), which adapted on Olympus DP70 model microscope (Olympus Optical, Tokyo, Japan). Measurements were carried out with UTHSCSA Image Tool for Windows Version 3.00 software.
The consecutive 10 sections were stained with toluidine blue and the other 10 sections were stained with periodic acid-Schiff (PAS). Photomicrographs were randomly taken from five fields of each section which were stained with toluidine blue. For mast cell enumeration, a standard transparent counting frame representing an area of 16,400μm2 was manually used and eight fields in each photograph were examined for each mouse. Goblet cells stained with PAS were enumerated in 10 sections of each mouse. In each section, three to five randomly selected airways were photographed. Circumferences of all airways were measured and goblet cell numbers in these areas were recorded. For standardisation, goblet cell numbers in 100μm were analysed by dividing the total goblet cell number to the total length of airway circumferences and multiplying the result by one hundred.
Measurement of cytokines in lung homogenatesLungs were removed and washed with cold PBS three times in order to remove the blood. Lung tissues were taken into 2ml microcentrifuge tubes and stored at −80°C until analysis. On the study day, frozen lung tissues were thawed, weighed (60–80mg), transferred into different tubes on ice containing 5ml of stainless beads, 0.1% SDS protease inhibitor cocktail (Sigma–Aldrich, St. Louis, MO, USA) and 0.1mg/ml phenylmethanesulfonyl fluoride (PMSF) in PBS. Microcentrifuge tubes were transferred to pre-cooled Tissuelyser LT racks and placed into Tissuelyser (Qiagen, Germany) homogenisator. Time and frequency were set to 5 and 50min, respectively. The homogenates were then centrifuged at 15,000×g for 1h at 4°C and supernatants were obtained. Levels of IL-4, IL-5, IL-13 cytokines were quantified in the supernatants of the lung tissue by standard ELISA protocols by using commercial Mouse IL-4, IL-5, IL-13 (eBioscience Mouse ELISA kit, USA). Detection levels were 4pg/ml for IL-4 and IL-5, 2.8pg/ml for IL-13.
Measurement of NO in lung homogenatesNitric oxide levels were measured by ELISA in accordance with the manufacturer's recommendations in supernatants obtained from the lung tissue homogenates (Invitrogen Griess Reagent). Griess Reagent (A: 1-naphthyethylene-diamine dihydrochloride, 0.1%; B: sulfanilic acid 1%) and samples were mixed with an equal volume in microtiter plates (Greiner) and they were stored at room temperature for 30min. Results were determined at 548nm (BioTek Synergy HT, USA) using spectrophotometric methods.16
Measurement of serum OVA specific IgE in serumSerum levels of OVA specific IgE were measured using the ELISA commercial kit (Sun Red Biological Technology, Shanghai, China).
Immunohistochemical detectionsCaspase-3 (AB3623, Millipore, Temecula, CA, Polyclonal antibody), TUNEL (DeadEnd Colorimetric TUNEL system kit, Roche, Germany), MMP9 (anti-MMP-9 mouse monoclonal antibody, Santa Cruz Biotech, Inc. Germany), VEGF (anti-VEGF mouse monoclonal antibody, Santa Cruz Biotech, Inc. Germany), TGF-β (anti-TGF-β mouse monoclonal antibody, Santa Cruz Biotech, Inc. Germany) antibodies were used in immunohistochemistry experiments. After deparaffinisation and rehydration, sections were treated with trypsin (Cat No: 00-3008 Digest All 2A, Zymed, San Francisco, CA, USA) at 37°C for 15min (for TUNEL, MMP9, VEGF, TGF-β antibodies). For Caspase-3, samples were treated with 10mM citrate buffer (Cat No. AP-9003-125 Labvision) for five minutes to unmask antigens by using the heat treatment. Slides were cooled in buffer for 20min. For all sections, sections were incubated in a solution of 3% H2O2 for 15min and then with normal serum blocking solution in order to inhibit endogenous peroxidase activity. Sections were again incubated in a humid chamber for 18h at +4°C with primer antibodies, thereafter with biotinylated IgG, and then with streptavidin conjugated to horseradish peroxidase for 15min according to kit instructions (85-9043, Invitrogen Corporation, Camarillo, UK). Sections were finally stained with DAB (diaminobenzidine) (1718096, Roche, Mannheim, Germany) and counter-stained with Mayer's haematoxylin. Samples were analysed using a light microscope.17
Semi-quantification of immunostaining dataSemi-quantitative grading system was used to score the quantity of primer antibody positive staining in the sections.18 The score was defined as follows: 0: no immunoreactivity; 1: remarkably little positive staining; 2: moderate positive staining; 3: strong positive staining evenly distributed across the whole image. Each section was graded by two individuals blind to the treatments, and the average score was taken.
Statistical analysisAll values were expressed as the mean±standard deviation (SD). The Kruskal Wallis (between all groups) and Mann–Whitney U-test (for two groups) were used to compare staining intensity values between groups. All statistical analyses were performed using the SPSS software for Windows, Version 15.0 SPSS, Chicago, IL, USA. The significance was accepted when the p value was lower than 0.05 (p<0.05).
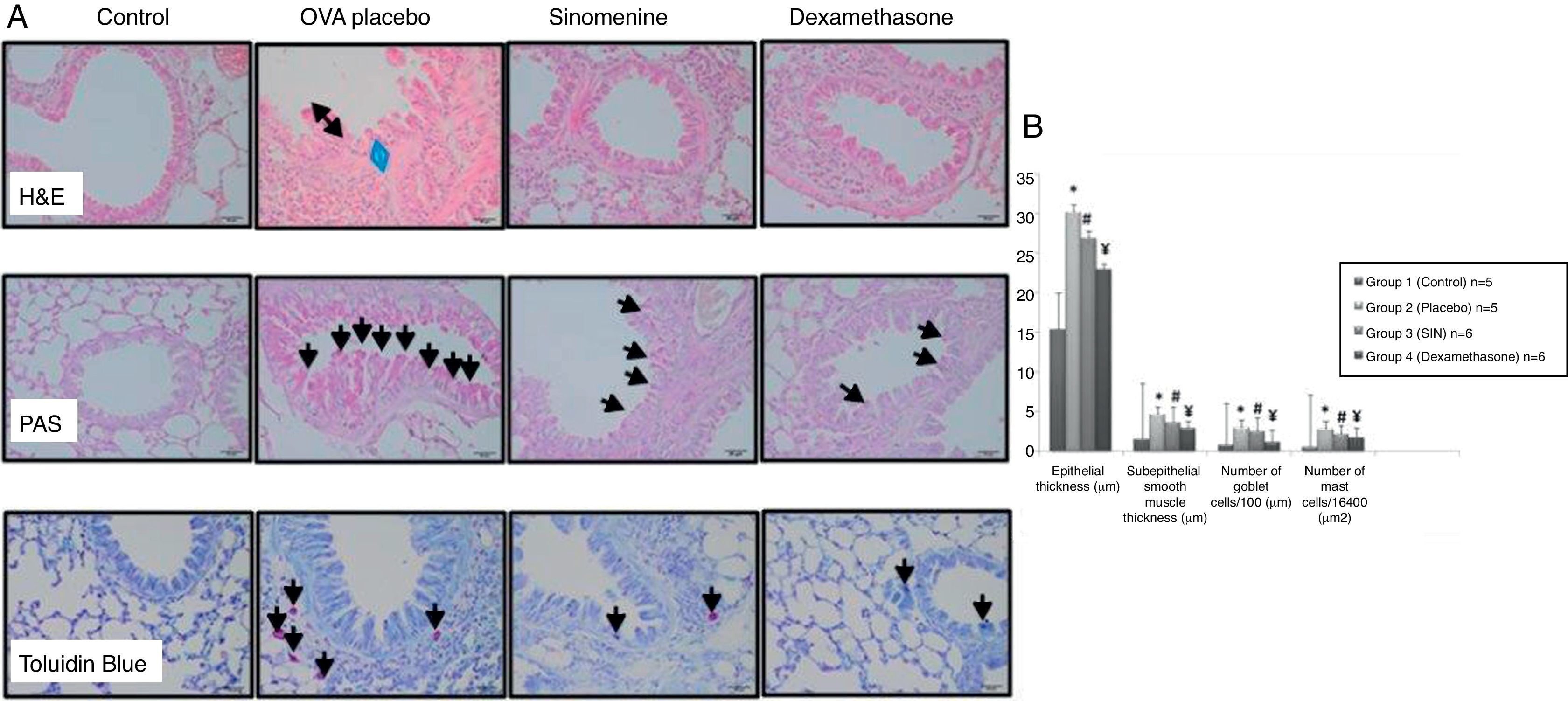
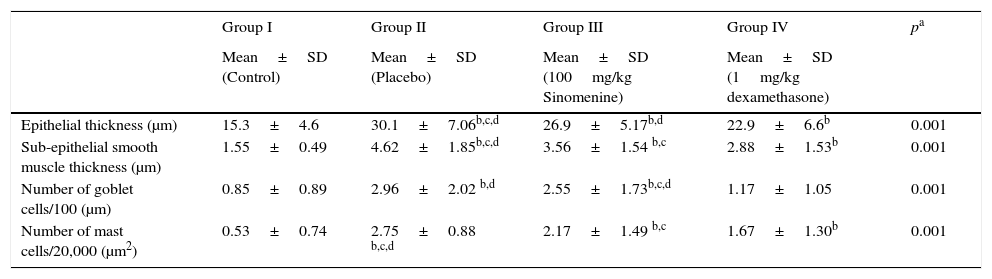
ResultsComparison of the histopathological findings of the study groupsThe light microscopic examinations revealed normal findings in the control group (Table 1). We compared the histopathological features of the control group with the placebo group (group II) to show that the asthma model was successfully established. In the placebo group (group II), thicknesses of epithelium and smooth muscle layers as well as numbers of mast cells and goblet cells were significantly higher compared to the control group (group I). These results showed that the model of chronic asthma was established.
Comparison of the histopathological findings of the study groups (mean±SD).
| Group I | Group II | Group III | Group IV | pa | |
|---|---|---|---|---|---|
| Mean±SD (Control) | Mean±SD (Placebo) | Mean±SD (100mg/kg Sinomenine) | Mean±SD (1mg/kg dexamethasone) | ||
| Epithelial thickness (μm) | 15.3±4.6 | 30.1±7.06b,c,d | 26.9±5.17b,d | 22.9±6.6b | 0.001 |
| Sub-epithelial smooth muscle thickness (μm) | 1.55±0.49 | 4.62±1.85b,c,d | 3.56±1.54 b,c | 2.88±1.53b | 0.001 |
| Number of goblet cells/100 (μm) | 0.85±0.89 | 2.96±2.02 b,d | 2.55±1.73b,c,d | 1.17±1.05 | 0.001 |
| Number of mast cells/20,000 (μm2) | 0.53±0.74 | 2.75±0.88 b,c,d | 2.17±1.49 b,c | 1.67±1.30b | 0.001 |
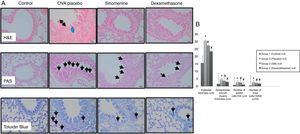
In 100mg/kg SIN treated group (group III), all histological parameters including sub-epithelial smooth muscle and epithelial thicknesses, number of mast cells except the number of goblet cells, were significantly better compared to the placebo (group II) and improvement was similar to the dexamethasone-treated group (group IV) in all parameters except sub-epithelial smooth muscle thickness and numbers of mast cells. All histological parameters were significantly better in the dexamethasone-treated group (group IV) compared to the placebo group (group II) (Table 1, Fig. 2A and B).
(A) Histopathological findings of study groups. I; control (n:5), II; placebo (n:5), III; 100mg/kg SIN (n:6), IV; dexamethasone (n:6) A; H&E, B; PAS, C; Toluidine Blue staining. (A) Arrow with two heads; epithelial thickness (black arrow), sub-epithelial smooth muscle thickening (blue arrow), (B) arrows; goblet cells, (C) arrows; mast cells. (B) Comparison graphics of the histopathological findings of the study groups.
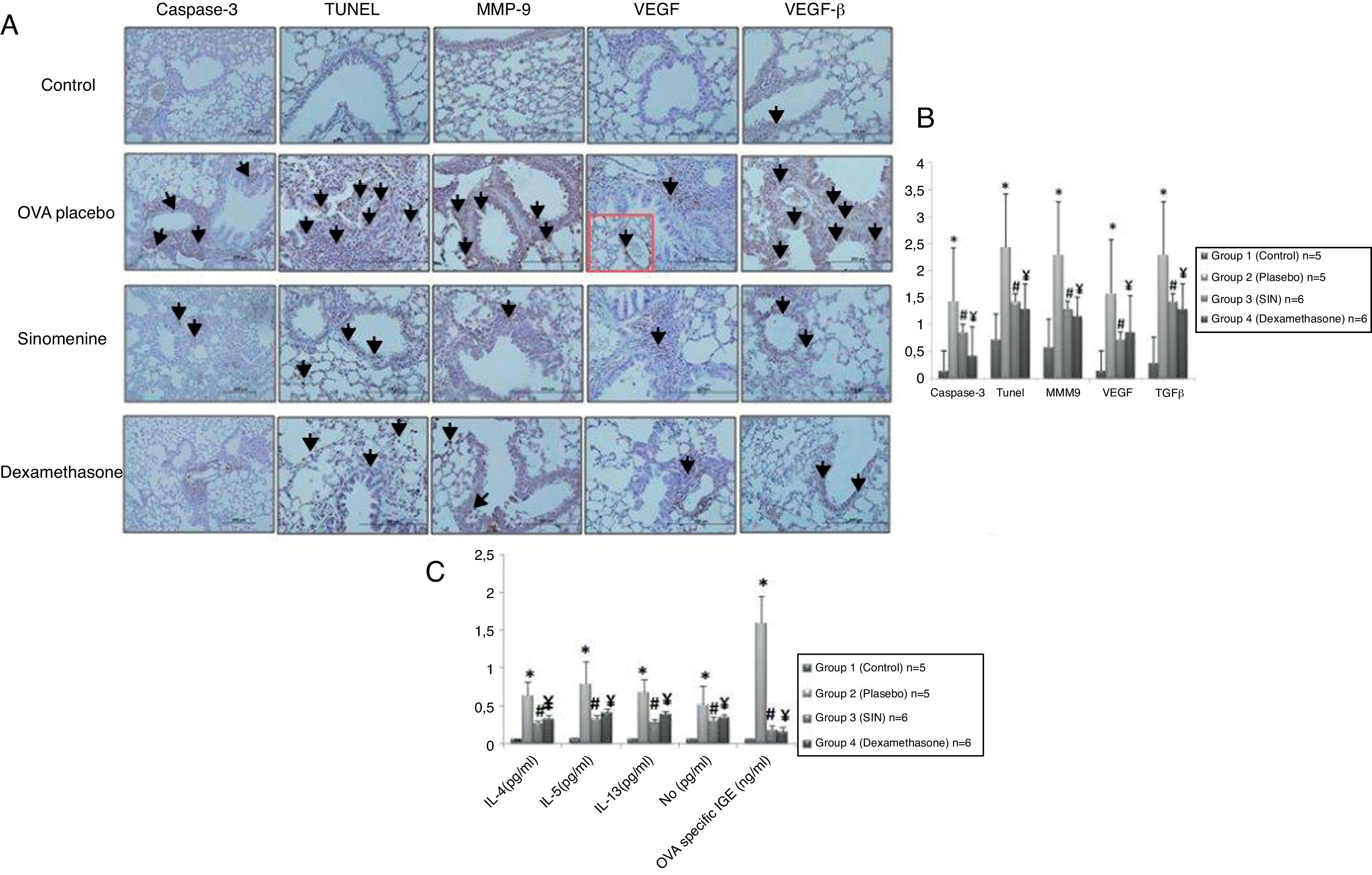
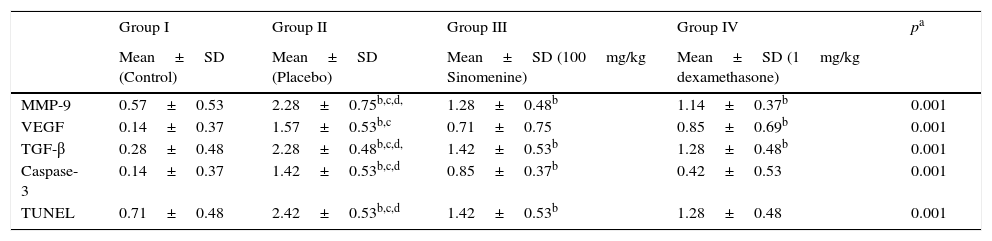
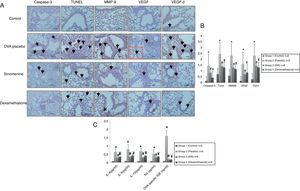
Immunohistochemical scoring of MMP-9, VEGF, TGF-β, TUNEL and caspase-3 were found lower in the control group (group I) compared to the placebo group (group II). In 100mg/kg SIN treated group, all the immunohistochemical scores of MMP-9, VEGF, TGF-β, TUNEL and caspase-3 were lower than the placebo group (group II). Dexamethasone treatment led to lower immunohistochemical scoring of MMP-9, TGF-β, TUNEL and caspase-3 except the VEGF, compared to the placebo group (group II). We also observed that immunohistochemical scoring of 100mg/kg SIN (group II) and dexamethasone treated groups (group IV) were similar in all parameters (Table 2, Fig. 3A and B).
Comparison of the immunohistochemical scoring of the study groups (mean±SD).
| Group I | Group II | Group III | Group IV | pa | |
|---|---|---|---|---|---|
| Mean±SD (Control) | Mean±SD (Placebo) | Mean±SD (100mg/kg Sinomenine) | Mean±SD (1mg/kg dexamethasone) | ||
| MMP-9 | 0.57±0.53 | 2.28±0.75b,c,d, | 1.28±0.48b | 1.14±0.37b | 0.001 |
| VEGF | 0.14±0.37 | 1.57±0.53b,c | 0.71±0.75 | 0.85±0.69b | 0.001 |
| TGF-β | 0.28±0.48 | 2.28±0.48b,c,d, | 1.42±0.53b | 1.28±0.48b | 0.001 |
| Caspase-3 | 0.14±0.37 | 1.42±0.53b,c,d | 0.85±0.37b | 0.42±0.53 | 0.001 |
| TUNEL | 0.71±0.48 | 2.42±0.53b,c,d | 1.42±0.53b | 1.28±0.48 | 0.001 |
MMP-9, matrix metallo proteinase-9; VEGF, vascular endothelial growth factor; TGB β, transforming growth factor-β; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick endlabeling and Caspase-3, cysteine-dependent aspartate-specific proteases.
(A) Immunohistochemical staining results of study groups. I; control (n:5), II; placebo (n:5), III; 100mg/kg SIN (n:6), IV; dexamethasone (n:6). (A); Caspase-3, (B); TUNEL, (C); MMP-9 (D); VEGF (E); TGF-β. Black arrows show positive staining with caspase 3, TUNEL, MMP-9, VEGF and TGF- β respectively. VEGF, vascular endothelial growth factor; MMP-9, matrix metallo proteinase-9, TGB-β, transforming growth factor-β, TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick endlabeling and Caspase-3, cysteine-dependent aspartate-specific proteases. (B) Comparison graphics of the immunohistochemical staining findings of the study groups. *p<0.05 vs. Group I, Group III, Group IV, # and ¥p<0.05 vs. placebo. (C) Comparison graphics of the cytokine levels of the study groups. *p<0.05 vs. Group I, Group III, Group IV, # and ¥p<0.05 vs. placebo.
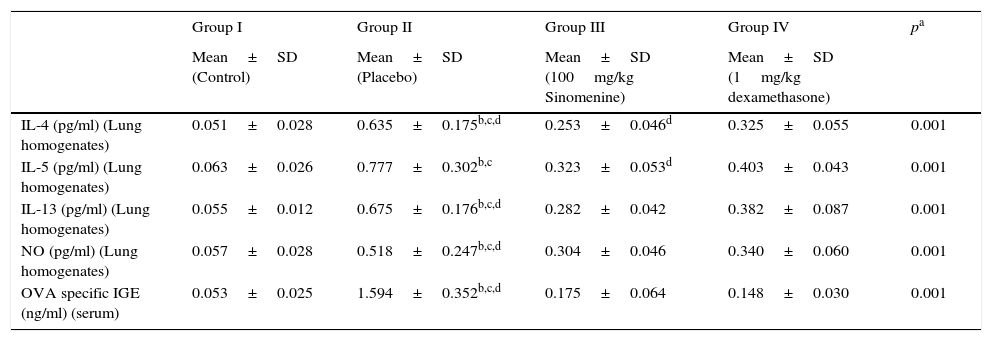
IL-4, IL-5, IL-13, NO levels in lung homogenates and OVA specific IgE levels in serum samples were significantly lower in control group (group I) compared to the placebo group (group II).
All cytokine levels (IL-4, IL-5, IL-13, NO) in lung homogenates and OVA-specific IgE level in serum samples were significantly lower in 100mg/kg SIN treated group (group III) compared to the placebo group (group II). All cytokine levels in lung homogenates and serum except the IL-5 were significantly lower in the dexamethasone-treated group compared to the placebo group (group II).
All cytokine levels except the IL-4 and IL-5 were similar in 100mg/kg SIN (group III) and dexamethasone (group IV) treated groups. In the dexamethasone-treated group (group IV) IL-4 and IL-5 levels were significantly lower than the 100mg/kg SIN treated group (group II) (Table 3, Fig. 3C).
Comparison of cytokine levels in lung homogenates and OVA-Specific IgE in Serum (mean±SD).
| Group I | Group II | Group III | Group IV | pa | |
|---|---|---|---|---|---|
| Mean±SD (Control) | Mean±SD (Placebo) | Mean±SD (100mg/kg Sinomenine) | Mean±SD (1mg/kg dexamethasone) | ||
| IL-4 (pg/ml) (Lung homogenates) | 0.051±0.028 | 0.635±0.175b,c,d | 0.253±0.046d | 0.325±0.055 | 0.001 |
| IL-5 (pg/ml) (Lung homogenates) | 0.063±0.026 | 0.777±0.302b,c | 0.323±0.053d | 0.403±0.043 | 0.001 |
| IL-13 (pg/ml) (Lung homogenates) | 0.055±0.012 | 0.675±0.176b,c,d | 0.282±0.042 | 0.382±0.087 | 0.001 |
| NO (pg/ml) (Lung homogenates) | 0.057±0.028 | 0.518±0.247b,c,d | 0.304±0.046 | 0.340±0.060 | 0.001 |
| OVA specific IGE (ng/ml) (serum) | 0.053±0.025 | 1.594±0.352b,c,d | 0.175±0.064 | 0.148±0.030 | 0.001 |
OVA, ovalbumin; IL, interleukin; NO, nitric oxide.
The number of eosinophils in BAL fluid was significantly reduced in the 100mg/kg SIN (group III) treated group when compared to the Placebo group (Table 4).
DiscussionAsthma is a common chronic disease of the airway that is characterised by the complex interaction of airway inflammation, airway remodelling, and bronchial hyperresponsiveness which leads to recurrent episodes of wheezing, coughing, breathlessness and chest tightness. Airway inflammation is typically eosinophilic and accompanied by elevation of Th2 cytokines. Airway remodelling is pathologically characterised by sub-epithelial thickening from increased deposition of extracellular matrix proteins such as collagens, goblet cell hyperplasia, increased airway smooth muscle thickness, angiogenesis.19,20
Sinomenine (SIN) is an alkaloid that is isolated from the root and stem of the climbing plant S. acutum. Previous reports have demonstrated that SIN has a wide range of pharmacological actions, including antirheumatic, anti-inflammatory, analgesic, antiarrhythmic, anti-angiogenesis and immunosuppressive effects.5–10 SIN has been used in the treatment of rheumatoid arthritis, glomerular diseases, and ventricular arrhythmia in clinical trials with relatively few side effects and favourable therapeutic effects.21
In a few studies, SIN has also been reported to have an anti-allergic effect.11–13 However, its related mechanisms underlying anti-allergic functions have not been fully elucidated. In this study, we investigated the effects of Sinomenine on airway remodelling, airway epithelial cell apoptosis and TH-2 immune responses in a murine model of chronic asthma, in comparison with the conventional dexamethasone treatment. In our study, we also demonstrated that 100mg/kg SIN treatment had beneficial effects on airway remodelling including epithelial thickness, sub-epithelial smooth muscle thickness and goblet cell hyperplasia. Furthermore, we also indicated the regulation of Th-2 immune response which led to lower levels of OVA specific IgE in serum and samples IL-4, 5, 13, NO in lung homogenates.
Apoptosis, or programmed cell death, is defined as an active physiological process of cell self-destruction involving specific morphological and biochemical changes. Caspase-3 is a critical executioner of apoptosis in the caspase signalling pathway. Moreover, caspase-3 is the key caspase responsible for the majority of apoptotic effects.22 SIN has been shown to modulate caspase activation to induce apoptosis in various types of cells.23–26 However, there is currently no report about the effect of SIN on the apoptosis of airway epithelial cells. Therefore, in our study we investigated the effects of SIN on the airway epithelial cell apoptosis in a murine model of chronic asthma. Unlike previous studies, we indicated that 100mg/kg doses of SIN treatment inhibited airway epithelial cell apoptosis compared to the placebo, which was demonstrated by using TUNEL and caspase-3 staining technique.
Angiogenesis is seen in asthma as a part of remodelling in association with a greater expression of vascular endothelial growth factor.27 Vascular Endothelial Growth Factor, a mediator derived from endothelial cells, but also from most inflammatory cells in asthma, plays a primary role in vascular remodelling and angiogenesis.28 In a previous study Kok et al. found that SIN exerts the anti-angiogenic effect in vitro and in vivo possibly through the inhibition or interruption of endothelial cell migration, proliferation, invasion and also morphological differentiation in extrapulmonary tissues.9 Our results showed that 100mg/kg doses of SIN treatment significantly decreased immunohistochemical scoring of VEGF in the lung tissue of asthmatic mice compared to placebo. So, we thought that SIN also had antiangiogenic activity in lung tissue of asthmatic mice.
Matrix metalloproteinases, especially matrix metalloproteinase-9 (MMP-9), are extracellular proteases which degrade the extracellular matrix (ECM) during the remodelling of tissues. Han et al. indicated that MMP-9 was expressed by bronchial epithelium and it may be an important factor for airway eosinophil infiltration in asthma subjects.29 In a previous study Zhou et al. demonstrated that, SIN suppresses the production of proinflammatory cytokines IL-1β and IL-6 in serum, inhibits protein expression and activities of MMP-2 and MMP-9 in rat paw tissues.30 Another study also suggested that SIN can suppress IL-1β-induced mRNA and protein expressions of MMP-1, MMP-3, MMP-9, and MMP-13 in SW1353 cells and human osteoarthritic (OA) chondrocytes.31 In our study, we have shown that 100mg/kg doses of SIN treatment significantly decreased immunohistochemical scoring of MMP-9 in the lung tissue of asthmatic mice compared the placebo.
Transforming growth factor-beta (TGF-beta) is an important fibrogenic and immunomodulatory factor. TGF-beta is believed to play an important role in most of the cellular biological processes leading to airway remodelling. This cytokine is produced by a number of cells, including macrophages, eosinophils, epithelial cells and fibroblasts and the concentration of TGF-beta correlates with disease severity.32 In a previous study, Liu et al. demonstrated that SIN markedly inhibited TGF-beta levels in an experimental antigen-induced arthritis model in rats. In another study, Feng et al. suggested that SIN have suppressive effects on both TH1 and TH2 immune responses but it enhanced the secretion of TGF-beta in mice.33 In our study, we have shown that 100mg/kg doses of SIN treatment significantly decreased immunohistochemical scoring of TGF-beta in the lung tissue of asthmatic mice compared the placebo.
With relatively few side effects and favourable therapeutic effects, SIN has been used in the treatment of various diseases, particularly rheumatoid arthritis, glomerular diseases, and ventricular arrhythmia in clinical trials.34 Clinical side effects encountered with high doses of injected SIN were: injection site flare, pruritus in the head and upper part of the body, oedema around the lips and eyelids, and temporary cephalalgia. Most of these side effects were reduced by classical antihistamines (H1-receptor antagonists).35 However, further studies are required about pharmacological effects and clinical applications for asthma.
There are some limitations to our study. We could not define the molecular mechanisms of SIN on airway epithelium and we did not use any method for the evaluation of airway hyperresponsiveness. Instead, we histopathologically and serologically assessed the effects of SIN on airway inflammation, epithelial apoptosis and remodelling.
In conclusion, taking all those data together, we found that SIN exerts the anti-asthmatic effect possibly through the inhibition TH2 immune response, apoptosis of airway epithelial cells and airway remodelling. The ability of SIN to modulate chronic asthma histopathology makes it a promising target for asthma therapy. However, further, more detailed basic and clinical studies are needed for the evaluation of SIN treatment in allergic airway diseases.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestWe, the authors of the manuscript, do not have any conflict of interest.
We thank all authors of the study. All procedures involving animal experiments which were performed in the study were in accordance with the ethical standards of the institution.