Cow's milk protein allergy (CMPA) is the most common food allergy in children worldwide. Some children have severe and persistent CMPA, with near-fatal reactions after exposure to trace amounts of cow's milk-proteins (CMP). Strict avoidance diet is difficult, negatively affects quality of life and represents a conservative approach. Therefore, different therapeutic strategies are necessary.
ObjectiveWe aimed to assess long-term efficacy and safety of oral immunotherapy (OIT) in children with severe and long-lasting IgE-mediated CMPA.
Materials and methodsThe authors present four case reports of patients with CMPA who underwent CMP-OIT, that have been under long-term follow-up up to nine years. We provide information about the clinical and laboratory evaluation. Skin prick tests (SPT), specific IgE and IgG4 were performed before, during, and after OIT. Immune profile after OIT was assessed by flow cytometry (lymphocyte subsets, regulatory T and B cells).
ResultsThe success rate was 100%, and all patients currently have a free diet with minimal diary ingestion of 200mL CMP or equivalent. Specific IgE levels and SPT to CMP have progressively decreased, and specific IgG4 levels have increased. CD4+CD25+CD127−/dim regulatory T cells were increased after OIT.
ConclusionsOIT ensured a clinical tolerance state after up to nine years, confirmed by both clinical and immune profile, allowing a diet without restrictions, with high satisfaction from patients and caregivers. We emphasize that OIT should be performed only by allergy experts in the hospital setting, and that only motivated families should be enrolled, since it is essential to ensure CMP daily intake at home.
Food allergy affects around 11–26 million Europeans, being more common in infancy and early childhood.1 The prevalence of food allergy is higher during the first few years of life, affecting up to 6% of children and 4% of adolescents and adults.2,3
Cow's milk protein allergy (CMPA) is the commonest food allergy in children worldwide.1 Although most patients have spontaneously recovery, recent data have shown that the number of children with persistent CMPA is becoming progressively higher. In fact, the actual numbers revealed have shown that resolution rates are low, being only 19, 42, 64, and 79% in children aged 4, 8, 12 and 16, respectively.4 Severe symptoms reported at the time of diagnosis are consistently related to a worse prognosis.1 The presence of asthma and/or rhinitis4,5 and higher levels of specific IgE have also proven to be risk factors for CMPA persistence.4,6
In Portugal, like in many other countries, food allergy is the main cause of pediatric anaphylaxis.7–10 Fatal accidents related to hidden allergen intake have occurred in patients with food allergy, especially among adolescents, where the risk of fatal reactions increases substantially as a result of decreased parental surveillance of their diet.11 CMPA presents itself as a very challenging entity in clinical practice and specific therapeutic options are sparse.
Currently, the induction of oral tolerance to a specific allergen has been increasingly referred as an effective therapeutic strategy, capable of modifying the natural history of the disease, conferring protection against inadvertent intake and, therefore, allowing a significant increase in quality of life. Over the last two decades, several publications about this therapeutic option and its different approaches emerged in the literature. Published randomized controlled studies reinforced the success of several oral immunotherapy (OIT) protocols to cow's milk (CM)12–14 as well as the persistence of their effect for several years after treatment15 mostly due to a maintained exposure to the implicated allergen.16 In addition, in 2017, the Spanish Society of Allergology and Clinical Immunology (SEAIC) published guidelines regarding CM and egg immunotherapy,17–19 and the European Academy of Allergy and Clinical Immunology (EAACI) has published guidelines on active treatment of food allergy with allergen immunotherapy.20,21
The authors present four case reports of patients with CMPA who underwent specific oral desensitization to CM (CM-OIT), that have been under a long-term follow-up up to nine years (from five to nine years duration). We aimed to assess the long-term efficacy and safety of CM-OIT in these patients, and to analyze their clinical (milk consumption and symptoms) and laboratory tests evolution during the described years of follow-up.
Materials and methodsThe four patients enrolled in this study were followed at the Immunoallergy Department and therefore all the information disposed is part of the clinical records. We present a summary of the clinical course of all of them. Further information of each patient is presented in Table 1.
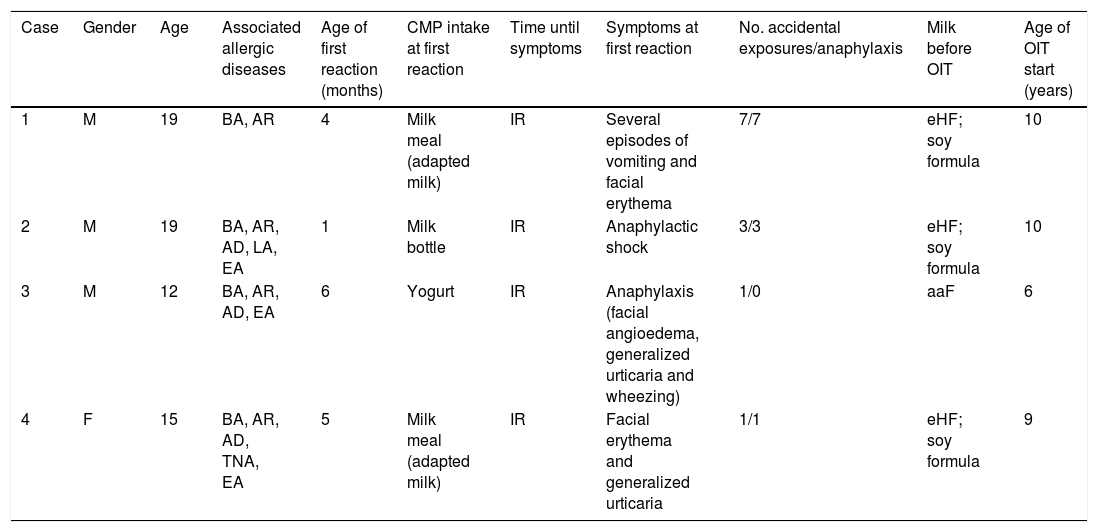
Clinical characterization of the CMPA patients enrolled in OIT protocol to CM.
| Case | Gender | Age | Associated allergic diseases | Age of first reaction (months) | CMP intake at first reaction | Time until symptoms | Symptoms at first reaction | No. accidental exposures/anaphylaxis | Milk before OIT | Age of OIT start (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 19 | BA, AR | 4 | Milk meal (adapted milk) | IR | Several episodes of vomiting and facial erythema | 7/7 | eHF; soy formula | 10 |
| 2 | M | 19 | BA, AR, AD, LA, EA | 1 | Milk bottle | IR | Anaphylactic shock | 3/3 | eHF; soy formula | 10 |
| 3 | M | 12 | BA, AR, AD, EA | 6 | Yogurt | IR | Anaphylaxis (facial angioedema, generalized urticaria and wheezing) | 1/0 | aaF | 6 |
| 4 | F | 15 | BA, AR, AD, TNA, EA | 5 | Milk meal (adapted milk) | IR | Facial erythema and generalized urticaria | 1/1 | eHF; soy formula | 9 |
aaF, aminoacid formula; AD, atopic dermatitis; BA, bronchial asthma; CM, cow's milk; CMP, cow's milk proteins; CMPA, cow's milk protein allergy; EA, egg allergy outgrown; eHF, extensively hydrolysed milk formula; F, female; IR, immediate reaction; LA, latex allergy; M, male; OIT, oral immunotherapy; RA, allergic rhinitis; TNA, tree nuts allergy.
Case 1: Male, 19 years-old, with asthma and allergic rhinitis. He was exclusively breastfed up to four months of age, when cow's milk-proteins (CMP) were introduced. At that age, CMPA was diagnosed after several episodes of vomiting and facial erythema after eating cereals with adapted milk. Despite CMP strict avoidance, at age of two years-old, he developed an episode of generalized urticaria and angioedema accompanied by laryngeal edema immediately after a meal with trace amounts of CMP in a restaurant, requiring epinephrine at emergency room. Afterwards, he had five more episodes of severe anaphylaxis, four of them after exposure to trace amounts of CMP, and one episode related to inhalation of CMP. In September 2008, at 10 years-old, in the presence of a severe and persistent IgE-mediated CMPA he was enrolled in a protocol of OIT to CM. The protocol was an adjustment of the protocol published by Martorell-Aragonés et al.22 and it was performed in the hospital setting. Most of the adverse reactions were promptly treated and self-limited, except for the anaphylactic reaction that occurred at the fourth day of the CM-OIT protocol, after a cumulative dose of 32mL. This was the seventh episode of anaphylaxis in his life, with generalized urticaria, peri-orbital, ears and penis angioedema, abdominal pain, rhinitis, conjunctivitis and wheezing, being promptly treated with intramuscular epinephrine and oral cetirizine and prednisolone, without further complications. For these reasons the protocol was adapted from five to nine days duration, always in the hospital setting. Follow-up: Since the end of the protocol the patient maintained a daily intake of 200mL of CM with progressive diet liberalization, being currently on the ninth year of follow-up. Clinical and laboratory evaluation are shown in Tables 2–4. Along these nine years of follow-up there was no need to decrease the CM daily dose, and the patient tolerates cow's, goat's and sheep's milk and dairy products.
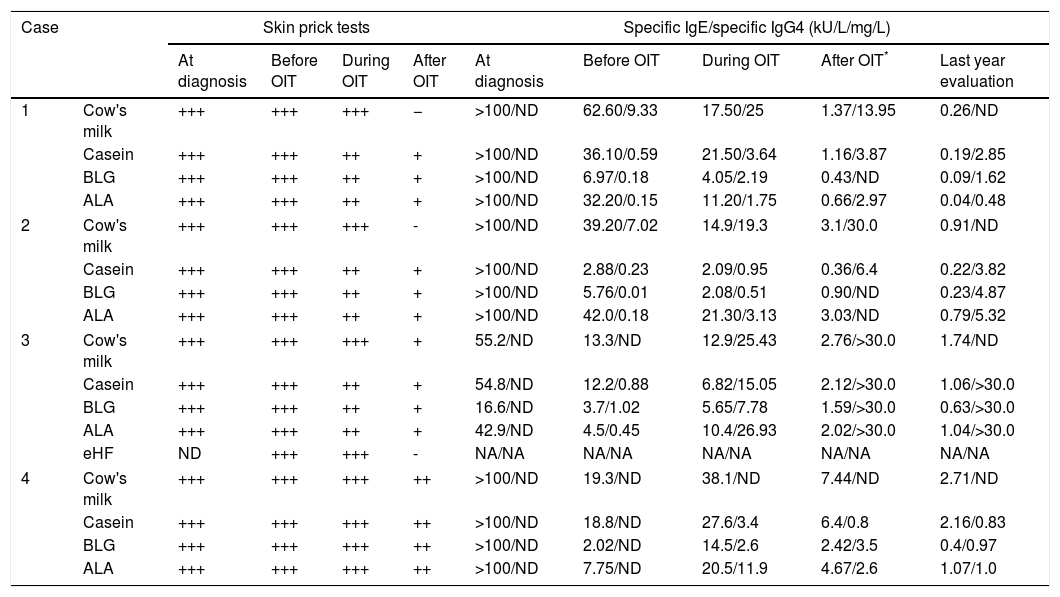
Diagnostic work-up of the CMPA patients enrolled in OIT protocol to CM.
| Case | Skin prick tests | Specific IgE/specific IgG4 (kU/L/mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| At diagnosis | Before OIT | During OIT | After OIT | At diagnosis | Before OIT | During OIT | After OIT* | Last year evaluation | ||
| 1 | Cow's milk | +++ | +++ | +++ | − | >100/ND | 62.60/9.33 | 17.50/25 | 1.37/13.95 | 0.26/ND |
| Casein | +++ | +++ | ++ | + | >100/ND | 36.10/0.59 | 21.50/3.64 | 1.16/3.87 | 0.19/2.85 | |
| BLG | +++ | +++ | ++ | + | >100/ND | 6.97/0.18 | 4.05/2.19 | 0.43/ND | 0.09/1.62 | |
| ALA | +++ | +++ | ++ | + | >100/ND | 32.20/0.15 | 11.20/1.75 | 0.66/2.97 | 0.04/0.48 | |
| 2 | Cow's milk | +++ | +++ | +++ | - | >100/ND | 39.20/7.02 | 14.9/19.3 | 3.1/30.0 | 0.91/ND |
| Casein | +++ | +++ | ++ | + | >100/ND | 2.88/0.23 | 2.09/0.95 | 0.36/6.4 | 0.22/3.82 | |
| BLG | +++ | +++ | ++ | + | >100/ND | 5.76/0.01 | 2.08/0.51 | 0.90/ND | 0.23/4.87 | |
| ALA | +++ | +++ | ++ | + | >100/ND | 42.0/0.18 | 21.30/3.13 | 3.03/ND | 0.79/5.32 | |
| 3 | Cow's milk | +++ | +++ | +++ | + | 55.2/ND | 13.3/ND | 12.9/25.43 | 2.76/>30.0 | 1.74/ND |
| Casein | +++ | +++ | ++ | + | 54.8/ND | 12.2/0.88 | 6.82/15.05 | 2.12/>30.0 | 1.06/>30.0 | |
| BLG | +++ | +++ | ++ | + | 16.6/ND | 3.7/1.02 | 5.65/7.78 | 1.59/>30.0 | 0.63/>30.0 | |
| ALA | +++ | +++ | ++ | + | 42.9/ND | 4.5/0.45 | 10.4/26.93 | 2.02/>30.0 | 1.04/>30.0 | |
| eHF | ND | +++ | +++ | - | NA/NA | NA/NA | NA/NA | NA/NA | NA/NA | |
| 4 | Cow's milk | +++ | +++ | +++ | ++ | >100/ND | 19.3/ND | 38.1/ND | 7.44/ND | 2.71/ND |
| Casein | +++ | +++ | +++ | ++ | >100/ND | 18.8/ND | 27.6/3.4 | 6.4/0.8 | 2.16/0.83 | |
| BLG | +++ | +++ | +++ | ++ | >100/ND | 2.02/ND | 14.5/2.6 | 2.42/3.5 | 0.4/0.97 | |
| ALA | +++ | +++ | +++ | ++ | >100/ND | 7.75/ND | 20.5/11.9 | 4.67/2.6 | 1.07/1.0 | |
ALA, alfalactoalbumin; BLG, betalactoglobulin; CM, cow's milk; CMPA, cow's milk protein allergy; eHF, extensively hydrolysed milk formula; NA, not applicable; ND, not done; OIT, oral immunotherapy.
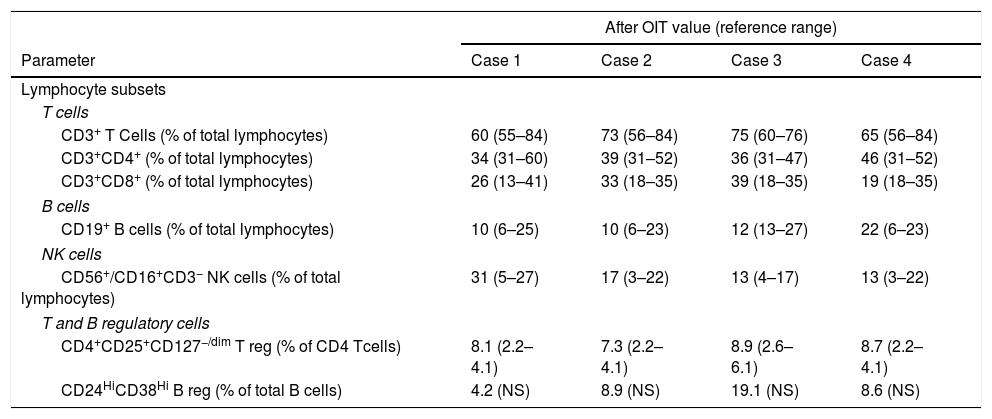
Immune profile of the CMPA patients enrolled in OIT protocol to CM.
| After OIT value (reference range) | ||||
|---|---|---|---|---|
| Parameter | Case 1 | Case 2 | Case 3 | Case 4 |
| Lymphocyte subsets | ||||
| T cells | ||||
| CD3+ T Cells (% of total lymphocytes) | 60 (55–84) | 73 (56–84) | 75 (60–76) | 65 (56–84) |
| CD3+CD4+ (% of total lymphocytes) | 34 (31–60) | 39 (31–52) | 36 (31–47) | 46 (31–52) |
| CD3+CD8+ (% of total lymphocytes) | 26 (13–41) | 33 (18–35) | 39 (18–35) | 19 (18–35) |
| B cells | ||||
| CD19+ B cells (% of total lymphocytes) | 10 (6–25) | 10 (6–23) | 12 (13–27) | 22 (6–23) |
| NK cells | ||||
| CD56+/CD16+CD3− NK cells (% of total lymphocytes) | 31 (5–27) | 17 (3–22) | 13 (4–17) | 13 (3–22) |
| T and B regulatory cells | ||||
| CD4+CD25+CD127−/dim T reg (% of CD4 Tcells) | 8.1 (2.2–4.1) | 7.3 (2.2–4.1) | 8.9 (2.6–6.1) | 8.7 (2.2–4.1) |
| CD24HiCD38Hi B reg (% of total B cells) | 4.2 (NS) | 8.9 (NS) | 19.1 (NS) | 8.6 (NS) |
CM, cow's milk; CMPA, cow's milk protein allergy; NS, not standardized; OIT, oral immunotherapy.
Reference range according age published by Shearer et al. and Van Gent et al.25,26
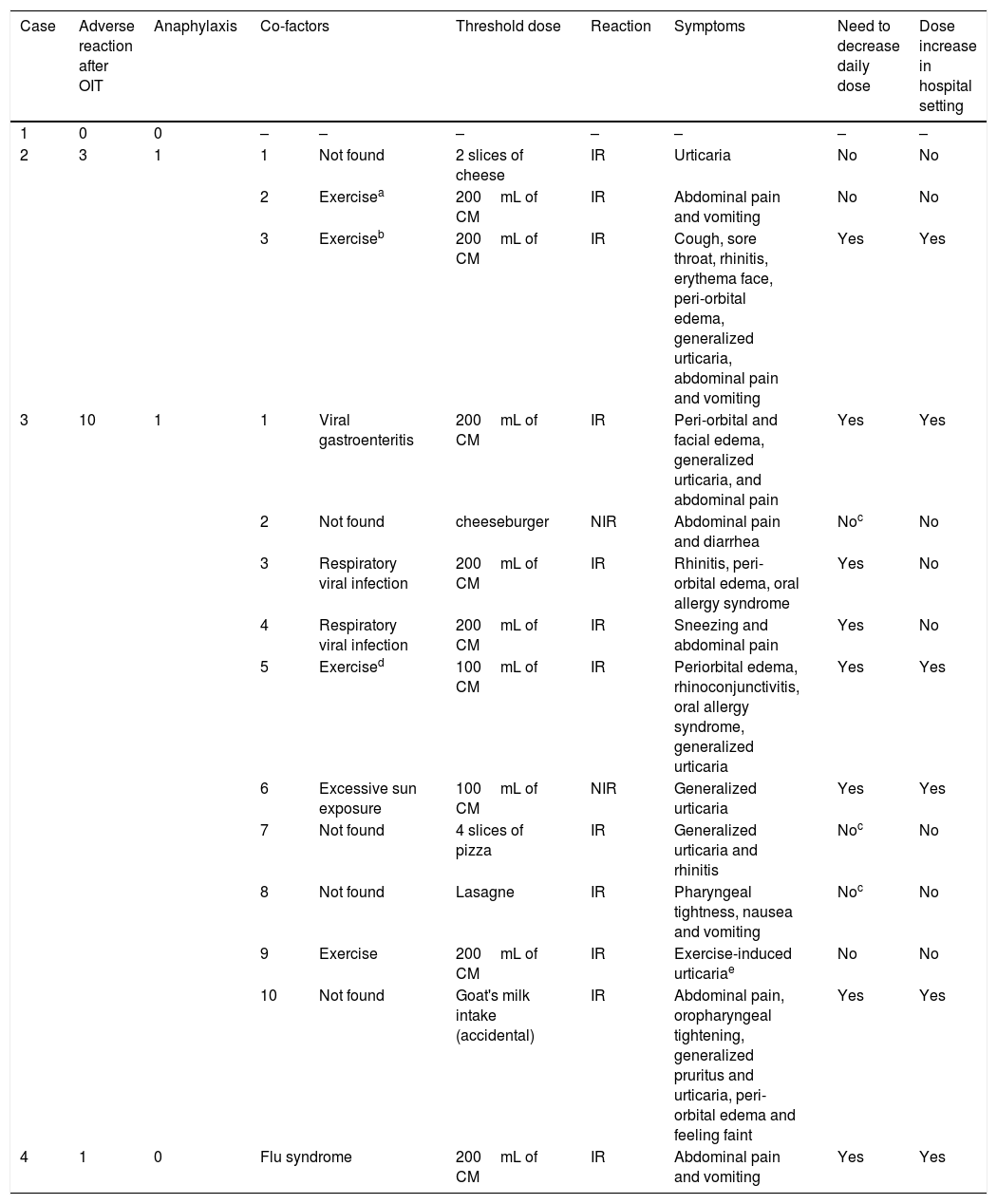
Clinical characterization of the patient's adverse reactions to CMP after conclusion of CM-OIT protocol.
| Case | Adverse reaction after OIT | Anaphylaxis | Co-factors | Threshold dose | Reaction | Symptoms | Need to decrease daily dose | Dose increase in hospital setting | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | – | – | – | – | – | – | – |
| 2 | 3 | 1 | 1 | Not found | 2 slices of cheese | IR | Urticaria | No | No |
| 2 | Exercisea | 200mL of CM | IR | Abdominal pain and vomiting | No | No | |||
| 3 | Exerciseb | 200mL of CM | IR | Cough, sore throat, rhinitis, erythema face, peri-orbital edema, generalized urticaria, abdominal pain and vomiting | Yes | Yes | |||
| 3 | 10 | 1 | 1 | Viral gastroenteritis | 200mL of CM | IR | Peri-orbital and facial edema, generalized urticaria, and abdominal pain | Yes | Yes |
| 2 | Not found | cheeseburger | NIR | Abdominal pain and diarrhea | Noc | No | |||
| 3 | Respiratory viral infection | 200mL of CM | IR | Rhinitis, peri-orbital edema, oral allergy syndrome | Yes | No | |||
| 4 | Respiratory viral infection | 200mL of CM | IR | Sneezing and abdominal pain | Yes | No | |||
| 5 | Exercised | 100mL of CM | IR | Periorbital edema, rhinoconjunctivitis, oral allergy syndrome, generalized urticaria | Yes | Yes | |||
| 6 | Excessive sun exposure | 100mL of CM | NIR | Generalized urticaria | Yes | Yes | |||
| 7 | Not found | 4 slices of pizza | IR | Generalized urticaria and rhinitis | Noc | No | |||
| 8 | Not found | Lasagne | IR | Pharyngeal tightness, nausea and vomiting | Noc | No | |||
| 9 | Exercise | 200mL of CM | IR | Exercise-induced urticariae | No | No | |||
| 10 | Not found | Goat's milk intake (accidental) | IR | Abdominal pain, oropharyngeal tightening, generalized pruritus and urticaria, peri-orbital edema and feeling faint | Yes | Yes | |||
| 4 | 1 | 0 | Flu syndrome | 200mL of CM | IR | Abdominal pain and vomiting | Yes | Yes | |
CM, cow's milk; CMP, cow's milk proteins; IR, immediate reaction; NIR, not immediate reaction; OIT, oral immunotherapy.
Case 2: Male, 19 years-old, with asthma, allergic rhinitis, atopic dermatitis, latex allergy due to multiple surgical interventions due to intestinal malrotation congenital malformation, and egg allergy outgrown at age of six years old. The first episode of CMPA was an anaphylactic shock after the first intake of CMP when he was one month old. He had two more anaphylactic episodes after accidental intake of CMP present in cereals with adapted milk and soya yogurt containing CMP, both requiring epinephrine at emergency room. In September 2008, at nine years of age he was enrolled in an OIT protocol to CM, since he had a severe and persistent IgE-mediated CMPA. We used the protocol cited for patient 1,22 also performed in the hospital setting. Most of the adverse reactions were self-limited with spontaneous regression, without medication. Follow-up: Since the conclusion of the protocol the patient maintained a daily intake of 200mL of CM with progressive diet liberalization. Along these nine years of follow-up, three reactions after accidental exposure occurred, one of which was anaphylaxis. The first episode occurred four months after the conclusion of the OIT protocol, consisting of urticaria after the intake of one hamburger containing two slices of cheese, which promptly remitted after cetirizine intake. The second episode occurred two years later, with abdominal pain and vomiting after exercise (long walk to the mall) immediately after CM intake, which resolved spontaneously. The third episode was a food-dependent exercise-induced anaphylaxis (FDEIA), in June 2017, at the age of 18 years old, after intense workout in less than one hour after the intake of 200mL of CM and was characterized by cough, sore throat, rhinitis, facial erythema, peri-orbital edema, generalized urticaria, abdominal pain and vomiting that remitted after the use of inhaled bronchodilator (formoterol with budesonide) and oral cetirizine and prednisolone. After this episode CM reintroduction was performed at hospital setting, without adverse reaction. Nowadays, the adolescent is on 200mL of CM daily consumption plus free diet, and he tolerates cow's, goat's and sheep's milk and dairy products. Clinical and laboratory evaluation are shown in Tables 2–4.
Case 3: Male, 12 years-old, with asthma, allergic rhinitis and atopic dermatitis. He had a personal history of egg allergy outgrown at the age of five years. He developed the first episode of anaphylaxis to CMP at the age of six months old, immediately after the intake of a yogurt, with facial angioedema, generalized urticaria, rhinoconjunctivitis and wheezing. He also had vomiting after eating cereals with adapted milk. The child began CMP avoidance and was put on an eHF (extensively hydrolyzed milk formula). However, several episodes of reproducible urticaria occurred immediately after eHF introduction. Due to positive skin prick test (SPT) to eHF and food protein-induced enterocolitis syndrome caused by soy milk the child was still on an aminoacid formula (Neocate®, Nutricia) at the age of six years. His parents reported the occurrence of episodes of contact urticaria to CM, but no episodes of accidental CM ingestion have been described. At the age of six years, an open oral provocation test with CM was performed, which was positive 17min after ingestion of 1mL of CM, with generalized urticaria, rhinoconjunctivitis and palpebral edema, treated with oral cetirizine and betamethasone. In June 2011, at six years of age he was enrolled to an OIT protocol to CMP, due to a severe and persistent IgE-mediated CMPA including eHF. The protocol was performed in the hospital setting according to the protocol published by Morais-Almeida et al.23 Although the present case has been previously published,24 no follow-up information has been disclosed thereafter. This was the first report of a patient with eHF allergy treated with OIT. Follow-up: Since the end of the protocol the patient maintained the daily intake of 200mL of CM and progressive liberalization of diet. Along the six years of follow-up there were several mild to moderate reactions, mainly with mucocutaneous or gastrointestinal symptoms, after daily CM intake, occurring in the presence of cofactors, such as exercise or during infections (acute gastroenteritis and respiratory infections), that lead to daily intake decrease (characterized in Table 4). Afterwards, every dose increasement has been performed in the hospital setting. There was an anaphylactic reaction, in September 2017, at the age of 12 years, after accidental intake of goat's milk, with abdominal pain, oropharyngeal tightening, generalized pruritus, urticaria, peri-orbital edema and faintness feeling that remitted after intramuscular epinephrine and oral cetirizine and prednisolone. Currently, the patient is on 200mL of CM daily consumption plus free diet, except for avoidance of goat's and sheep's milk and cheese. Clinical and laboratory evaluation are shown in Tables 2–4.
Case 4: Female, 15 years-old, with asthma and allergic rhinitis, atopic dermatitis, tree nuts allergy, egg allergy outgrown at age of 11 years-old, vitiligo and alopecia. The first symptoms appeared at the age of five months immediately after eating cereals with adapted milk with facial erythema and generalized urticaria. Despite CMP strict avoidance, at five years of age, she developed an anaphylactic reaction immediately after accidental intake of goat's milk, with oral allergy syndrome, facial erythema, generalized urticaria and laryngeal edema with respiratory distress, requiring epinephrine at the emergency room. In February of 2012, at the age of nine years, she was enrolled to an OIT protocol to CMP due to a severe and persistent IgE-mediated CMPA. We used the protocol cited for patient 3,23 also performed in the hospital setting. Most of the adverse reactions that occurred during the OIT protocol were mild and self-limited. Follow-up: Since the end of the protocol the patient maintained the daily intake of 200mL of CM with progressive liberalization of the diet. Along these five years of follow-up, at the age of 11 years, an episode of abdominal pain and vomiting occurred after the CM intake during a flu syndrome that lead to a decrease in the daily intake dose. Afterwards, dose increasement was performed in the hospital setting without adverse reactions. Nowadays, the adolescent is on 200mL of CM daily consumption plus free diet, and she tolerates cow's, goat's and sheep's milk and dairy products. Clinical characteristics and diagnostic procedures and results are presented in Tables 2–4.
Diagnostic work-upIn vivo testsSPT were the first step of the in vivo work-up. SPT to cow's milk and milk fractions (casein, alfalactoalbumin and betalactoglobulin) were performed with commercial extracts (Laboratories Leti™, Madrid, Spain) before, during, and after protocol, as shown in Table 2. When required SPT with eHF, goat's milk, sheep's milk and soy milk were performed. SPT were considered positive if the mean wheal diameter was 3mm or greater, with negative control (0.9% saline) and positive control (histamine 10mg/mL).
In vitro testsSerum-specific IgE and IgG4 antibodies (ImmunoCAP®, Thermo Fisher™ Scientific, Waltham, MA, USA) to cow's milk and milk fractions (casein, alfalactoalbumin and betalactoglobulin) were performed before, during, and after protocol, as shown in Table 2. For serum-specific IgE, a cut-off value ≥0.35kU/L was considered for positivity.
Immune profile after OIT was assessed by flow cytometry with a 4-color BD FACS Calibur® (BD Biosciences™, San Jose, CA, USA). Lymphocyte subsets were evaluated using the BD Multitest IMK Kit (BD Biosciences™) for the characterization of T cells, including CD4 and CD8 T cells, B cells and NK cells. Regulatory T (Tregs) and B cells (Bregs) were also analyzed and identified respectively as the CD4+CD25+CD127−/dim T cells, and the CD24HiCD38Hi B cells. Results are displayed in Table 3, including available reference ranges adjusted to age according to the studies by Shearer et al. and Van Gent et al.25,26
DiscussionOver the last two decades, several publications have emerged regarding OIT as a therapeutic option for CMPA.17–21 Nevertheless, literature concerning follow-up is scarce. In 2007, Martorell-Aragonés et al. published a three-year follow-up study.22 In 2016, Paassilta et al. published a work that included patients that have been followed up to seven years, by phone interviews.14 In the same year, Pajno et al. published a 10-year experience on OIT to milk and hen's egg, although few data were disclosed regarding the maximum years of follow-up for OIT to CM.27 Thus, there is still a lack of reports corroborating the long-term efficacy and safety of OIT protocols in daily basis clinical practice. In our study we could reach up to nine years of follow-up after OIT in four children with severe and long-lasting CMPA, by continuous clinical and laboratory evaluation. All of them have successfully completed the protocol and currently maintain CM daily intake plus CMP free diet with good tolerance.
However, we would like to enhance the role of comorbidities and co-factors for the occurrence of adverse reactions even several years after OIT. First of all, we must stress that all patients enrolled in this study had other allergic comorbidities (patient 3 was inclusively allergic to an eHF), and one of them had auto-immune comorbidities (patient 4 besides allergic comorbidities also had vitiligo and alopecia). Therefore, we emphasize the role of comorbidities like asthma or allergic rhinitis27 and we hypothesize that auto-immunity may constitute a potential constraint for a successful desensitization. Regarding the role of cofactors, according to the EAACI21 and Spanish guidelines19 published in 2017, some factors (fasting, exercise, infections, non-steroid anti-inflammatory medication use, menses, and milk irregular intake), may increase the risk of reactions during the maintenance phase of OIT to CMA. Some case reports have been published regarding FDEIA28 and food-dependent exercise-induced urticaria (FDEIU)29 after successful OIT protocols to CM.
FDEIA seems to play a role in accidental reactions even years after a successful OIT protocol. We highlight that our patient 2 suffered FDEIA related to CM eight years after OIT. Couto et al.29 also published in 2012 a FDEIU case report describing an adolescent that had a reaction several years after successful OIT to CM. It must be strengthened that these episodes are usually difficult to control since it is not easy in this age to plan what exercise will take place. Another important aspect is that we should always take into account the patients comments and feedback. For instance, our patient 3 consistently reported along these years that being tired (he was an athlete at the football league) or sick, made him more prone to react to foods containing CMP. Similarly, our patient 4 only had one reaction after the protocol, during a flu episode.
We ensure that all our patients had strict recommendations regarding avoidance of CM intake while fasting and to avoid physical exercise for at least two hours after CM intake (not only during the build-up phase but also during the OIT maintenance phase).17–19,30 All patients were also advised to keep the emergency prescription (including epinephrine auto-injector) with them all the time, but unfortunately patient 2 although with nine years of follow-up did not carry his epinephrine auto-injector with him in the FDEIA reported episode.
It is important to always ensure the control of coexisting allergic diseases, particularly asthma, during and after OIT. In situations of infections or febrile conditions and poorly controlled baseline allergic diseases there is an increased risk of adverse reaction to previously tolerated CM doses. So, in the presence of an asthma attack or an undercurrent disease like gastroenteritis or a febrile airway infection, a 50% reduction of the CM dose should be recommended in most severe patients not only during the build-up phase but also during the maintenance phase.17–19,30
It is very important to bear in mind that CM-OIT is species specific and does not guarantee tolerance to milk from other mammalian species due to the lack of cross-reactivity between caprine caseins and CM caseins.31 Consequently, once OIT has been completed, patient sensitization and evaluation of tolerance to goat's and sheep's milk are mandatory before their introduction into the diet.17–19,30 Our patients were also on avoidance of goat's and sheep's milk and cheese, but nowadays, except for patient 3, all of them can eat goat's and sheep's milk and dairy products. We emphasize that our patient 3 had an anaphylactic reaction after accidental intake of goat's milk six years after CM-OIT.
Regarding the diagnostic work-up of the CMPA patients enrolled in an OIT protocol to CM, as published by Martorell-Aragonés et al.,22 our results indicate that we have achieved clinical tolerance to CMP with our OIT protocol. We could demonstrate a progressively descendent profile of specific IgE to CM and CMP and a progressively ascendant profile of specific IgG4 to the same proteins.24,32 Moreover, we have also observed a reduction of SPT wheals on CM and CMP. Concerning peripheral regulatory T cell subsets, in these four patients we have found an increase in the population of CD4+CD25+CD127−/dim Tregs, which has been proven to be related to tolerance acquisition after immunotherapy and OIT protocols.33,34
It should be emphasized that OIT must be performed only by allergy experts in the hospital setting, and that only children with motivated caregivers should be enrolled, since it is essential to ensure the daily intake of CM at home.
We emphasize the importance of the patient–doctor relationship and the continuous channel of communication was a key for the success of these case series. Total availability of the clinical staff plays an exceptional role for the success of this treatment option as well as such for as avoiding drop-outs in OIT treatment. Thus, we hypothesize that our 100% rate of success may be in part due to a thigh connection and easy access and evaluation by our medical team. Despite the occurrence of adverse reactions during the years of follow-up we still consider OIT as an effective and safe option of treatment.
We recognize as a weakness of our study the lack of information regarding the gain in quality of life of our patients and families. Nevertheless, this was not considered as an aim of our study, and therefore we did not use any quality of life questionnaires before, during or after the OIT protocol. More studies should be done in order to quantify the gains in quality of life in this setting, although the advantages seem to be fare immensurable for both patients and families.20
Finally, it is also important to acknowledge that these patients and families are extremely motivated, which is the only way to guarantee the daily intake of CM at home along the years. We assume that the substantial achievements in quality of life are the cornerstone for this adherence.
ConclusionsWe have proven in our patients the presence of a sustained tolerance to CM after a specific desensitization to CMP in children and adolescents during a follow-up period of up to nine years. To our knowledge this is the first report on a daily basis of such a long-term follow-up, although with only four cases, with a continuous clinical and laboratory evaluation along the years with proven efficacy and safety, and complete adhesion. Adverse reactions can still occur even after several years, but well-prepared patients and families play a key role for the quick resolution of these situations. Proper management of comorbidities and strict avoidance of co-factors should not be forgotten. OIT to CM has proven to be a successful and safe therapy in our case series and therefore it has been incorporated in our Immunoallergy Department whenever suitable.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that the parents of the patient included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the parents of the patient mentioned in the article. The author for correspondence is in possession of this document.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Funding sourceThe authors declare that no funding was received for the present study.
Conflicts of interestThe authors have no conflict of interest to declare.
The authors would like to thank the patients and families involved in this study, without them this study would not be possible.