It is considered that farm areas protect young patients from allergy and asthma due to high exposure to endotoxins.
AimTo compare CD4+/CD25+ T-regulatory cells and forkhead transcription factor Foxp3 expression in asthmatic children allergic to house dust mites (HDM) living in rural and farm areas.
Materials and MethodsThis was a prospective analysis of 35 children living in farm areas (n=19) and rural areas (n=16), aged 8–16, with allergic rhinitis (allergic to dust mites) and newly diagnosed asthma. Surface molecule CD4+CD25+Foxp3+ expression on cultured PBMCs was estimated by flow cytometry using fluorophore-conjugated monoclonal antibodies in each patient.
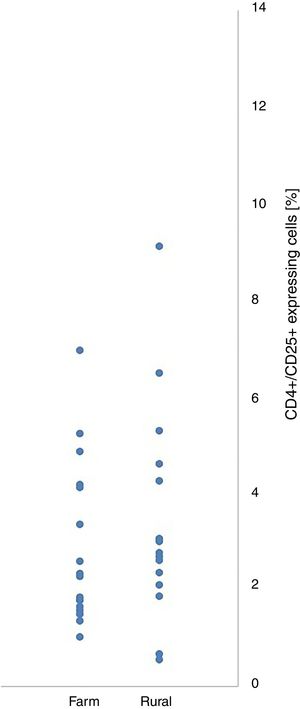
ResultsThirty-five children were included into the analysis: 19 children living in farm areas and 16 in rural areas. Within and between-groups (farm area vs. rural area) differences in CD4+/CD25+ and CD4+/CD25+Foxp3+ cell expression did not reach the level of significance.
ConclusionThe current analysis showed that CD4+/CD25+ and CD4+/CD25+Foxp3+ cell expression was not associated with place of living in asthmatic children sensitive to HDM.
On farms, there is high-level exposure to endotoxins (lipopolysaccharide (LPS), a cell wall component of Gram-negative bacteria. Environmental exposure to bacterial products has been found to be protective against allergy and asthma. In farm children, the prevalence of asthma and atopy is substantially lower.1 A study by Schuijs et al.,2 recently published in Science, showed that chronic exposure to endotoxin or farm dust protects mice from developing asthma. In the study by Kupryś et al.,3 researchers assessed the prevalence of asthma and atopy among children living in farms and among other children living in rural areas in Poland. They showed higher prevalence of asthma and allergic rhinitis in farm children compared to children from cities.
In our previous study, we compared the clinical effectiveness of immunotherapy (AIT) between children living in farm vs. rural areas4; treatment with immunotherapy resulted in overall clinical improvement, however more children living in farm areas responded better to AIT treatment based on the reduction in symptom-medication score. Studies have extended our understanding of the role of regulatory T-cells in immunotherapy. Environmental farm exposure is associated with higher regulatory T-cell numbers,2 while the precise mechanistic sequence of events remained elusive. Therefore, we compared CD4+/CD25+ TR cell and forkhead transcription factor Foxp3 expression in asthmatic children allergic to HDM living in rural and farm areas.
Materials and methodsThis was a prospective analysis of 35 children living in farm areas (n=19) and rural areas (n=16), aged 8–16, with allergic rhinitis (allergic to dust mites) and newly diagnosed asthma, who attended our allergy outpatient clinic in heating season of year 2016/2017 (November–April). There were no differences in asthma/allergic rhinitis severity between study groups. Children were considered as living in farm areas if they lived on a farm run by their family and have everyday contact with farm animals or animal feed. All other children were considered as living in rural areas (children living in the apartment/home without any animals at home or in the home yard). Asthma/allergic rhinitis diagnosis was defined previously by the doctors according to GINA and ARIA guidelines.5,6
The study was approved by the Ethical Committee of Medical University of LODZ, Lodz, Poland and a written consent was obtained from all the mothers before commencement of the study.
Immunological assessmentTregs were assessed using anti-CD25 (FITC-conjugated; Becton Dickinson, San Jose, CA, USA) anti-CD4 (PerCP-conjugated; Becton Dickinson, San Jose, CA, USA) and anti-human FOXP3 antibody monoclonal antibodies (PE-conjugated; Becton Dickinson, San Jose, CA, USA).
The peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by density gradient centrifugation on Pancoll human density-1077 (PAN Biotech, Germany) and cultured in standard mammalian cell culture conditions (37°C, 5% carbon dioxide, 80% relative humidity) for 72h, 2×106cells/mL in the presence of allergen in concentration 750SBU/mL. After cell culture cells were centrifuged and washed in the BD Pharmingen™ Stain Buffer (FBS) (5min/250g). Then they were incubated with 20μL of anti-CD25/anti-CD4 monoclonal antibody cocktail (for 20min, at room temperature, in the dark). Afterwards, cells were washed in BD Pharmingen™ Stain Buffer (FBS) and centrifuged (5min/250g). Cells were then re-suspended and incubated for 10min with Human FoxP3 Buffer A (at room temperature, in the dark). After washing the cells twice and discarding the supernatant, the sample was re-suspended in FoxP3 Buffer C. It was then incubated for 30min at RT protected from light. After washing the cells twice and discarding the supernatant, the sample was re-suspended in 20μL of PE anti-human FOXP3 or isotype control antibody was added and incubated for 30min (at room temperature, in the dark). After that, cells were washed twice, re-suspended with cell staining buffer, and the fluorescence was measured using flow cytometry (FACS CantoII, BD Biosciences, San Jose, CA, USA). Cells expressing CD4+/CD25high+/FOXP3+ were determined as Tregs. The percentage of Tregs among mononuclear cells, which were gated based on SSC and FSC distribution, was calculated.
Statistical methodsShapiro–Wilk test to assess normal distribution in studied population was used. During analysis of our data t-test for parametric variables and Mann–Whitney test for non-parametric variables for between-groups comparisons were used. All the statistical computations were carried out using the SPSS Statistics release 23. P-values at a level of P<0.05 were considered statistically significant.
ResultsThirty-five children were included in the analysis: 19 children living in farm areas (11 girls and 8 boys) and 16 in rural areas (8 girls and 8 boys). There were no differences between-groups in demographic data.
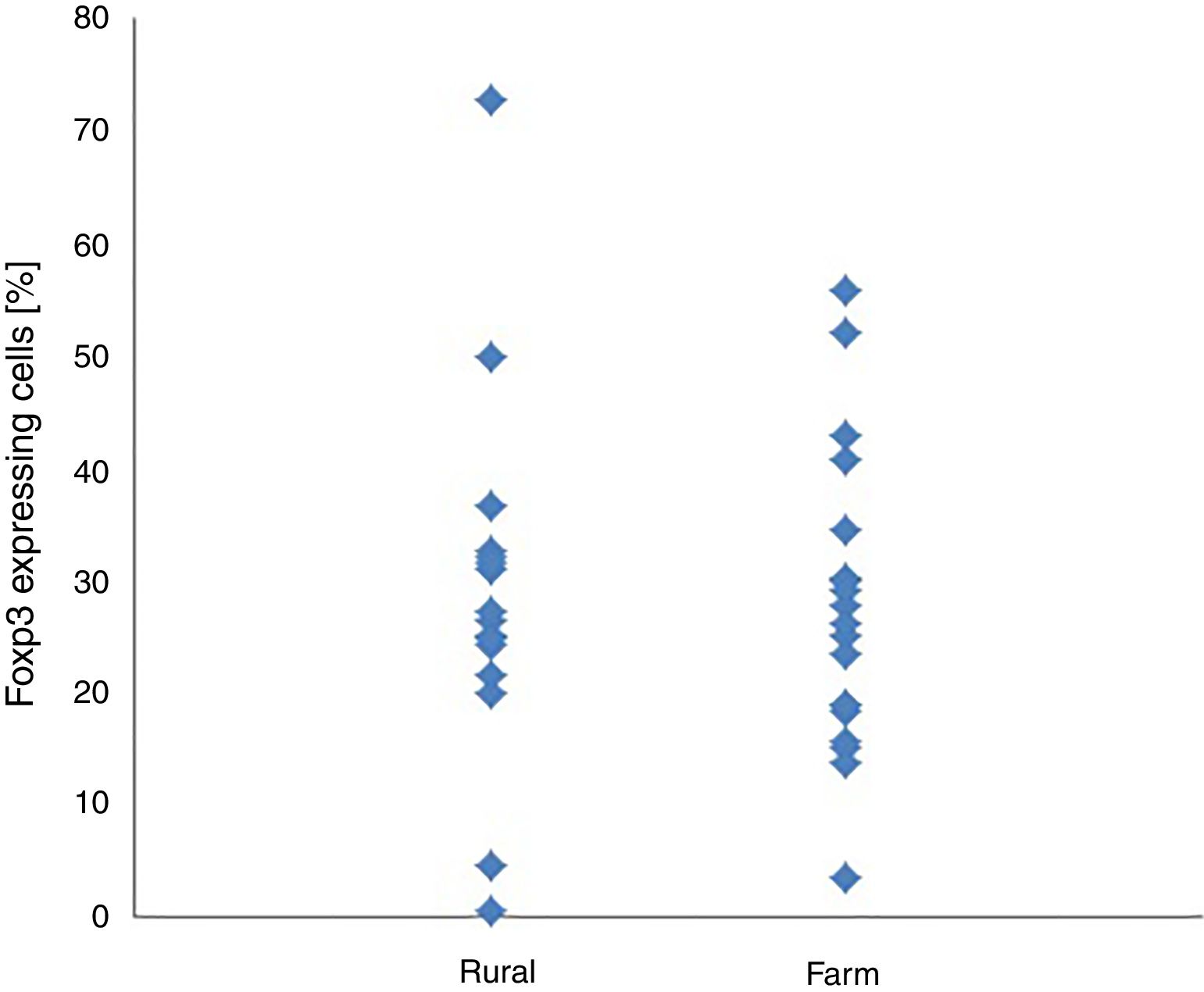
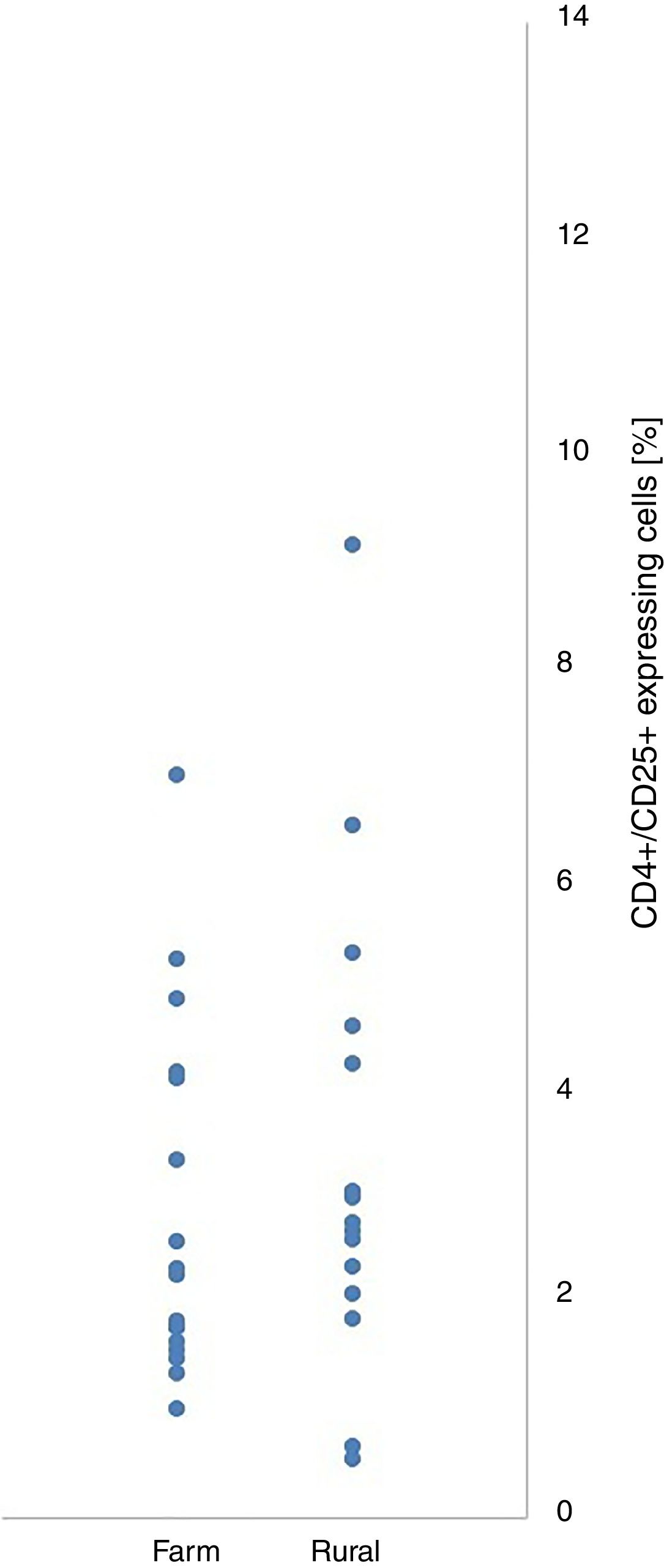
Within and between-groups (farm area vs. rural area) differences in CD4+/CD25+ and CD4+/CD25+Foxp3+ cell expression did not reach the level of statistical significance (Table 1). CD4+/CD25+ and CD4+/CD25+Foxp3+ expression were not associated with place of living of their patients (Figs. 1 and 2).
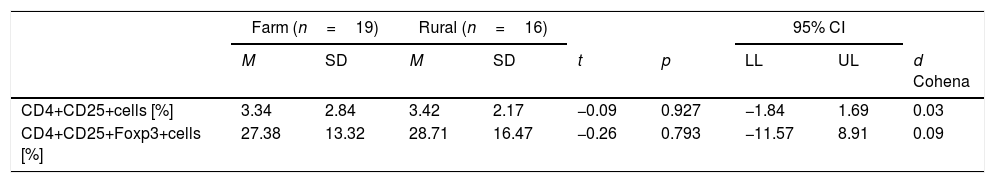
Differences between children living in farm areas and in rural areas in CD4+CD25+and CD4+CD25+Foxp3 cells expression in the presence of dust allergen.
| Farm (n=19) | Rural (n=16) | 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | t | p | LL | UL | d Cohena | |
| CD4+CD25+cells [%] | 3.34 | 2.84 | 3.42 | 2.17 | −0.09 | 0.927 | −1.84 | 1.69 | 0.03 |
| CD4+CD25+Foxp3+cells [%] | 27.38 | 13.32 | 28.71 | 16.47 | −0.26 | 0.793 | −11.57 | 8.91 | 0.09 |
n, number of children; M, mean; SD, standard deviation; t, Student t-test; P, significance; 95% CI, confidence interval; LL and UL, lower and upper limit of confidence interval.
Our study is the first showing the immunological profile of regulatory cells in allergic asthmatic children living in two environments. We showed that CD4+/CD25+ and CD4+/CD25+Foxp3+ expression was not associated with place of living of our patients.
After adjustment for the effect of demographic data we showed that living in farm areas was not independently associated with CD4+/CD25+ and CD4+/CD25+Foxp3+ expression.
Keeping in mind that the effectiveness of immunotherapy depended on the place of living4 we can hypothesize that the modification of immune regulation in sensitized children takes place during allergen immunotherapy, under environment interactions. Our findings are interesting especially in the context of the study by Schuijs et al.2 recently published in Science. They showed that chronic exposure to low-dose endotoxin or farm dust protects mice from developing house dust mite (HDM)-induced asthma which can result in lower prevalence of atopy in farm children.2 Our analysis showed that CD4+/CD25+ and CD4+/CD25+Foxp3+ cells expression was not associated with place of living in asthmatic children sensitive to HDM. Our observation was not described before in the literature. Also, for that reason we could not adequately estimate the power and sample size for our research. Therefore, we can only consider our results as a pilot study. We hypothesize that only pre-exposure to LPS protects against asthma through the epithelial production of A20 (an ubiquitin-modifying enzyme that attenuates NK-jB activation and release of GM-CSF) which was previously shown by Schujitsu et al.2 in mice. Given the limited statistical power of our study, this finding should be interpreted with caution before being replicated in a prospective large cohort study.
FundingThis study was funded by grant 503/6-029-01/503-61-001 and 503/6-029-01-503-61-001-17 from the Medical University of Lodz, Poland.
Conflict of interestThe authors have no conflict of interest to declare.