Lipid transfer proteins (LTPs) are panallergens found in many plant foods. They are a common cause of food-induced anaphylaxis (FIA) in adults living in the Mediterranean area. LTPs have also been proposed as a main cause of food-dependent exercise-induced anaphylaxis (FDEIA).
ObjectivesDescribe clinical characteristics and allergen sensitization profiles in patients with FIA related to LTP.
Materials and MethodsForty-three patients were included, aged 3–52 years with a clinical history of FIA and proven sensitization to LTP. Patients were tested with a multiple plant food and pollen panel and specific IgE to LTP allergens. LTP sensitization was assessed by in vivo (Pru p 3, LTP extract) and/or by in vitro tests (specific IgE, ImmunoCAP/ISAC®).
ResultsMedian age of first anaphylactic episode was 24 years (range 2–51), 44% had asthma, 74% were atopic and 42% had pollinosis (olive, mugwort, plane tree, wall pellitory and cypress). Co-sensitization to profilins was found in 22%. Overall in our center, LTP-induced anaphylaxis represents 17% of all causes of FIA. Foods implicated in anaphylactic reactions were: fresh fruits 51%, tree nuts 42%, vegetables (including peanut) 40% and seeds 14%. Seven patients had FDEIA.
ConclusionsLTPs are important allergens of FIA in Portugal. Clinical reactivity to several taxonomically unrelated plant foods may raise suspicion toward LTP sensitization. The association of LTP-induced anaphylaxis with pollinosis is relevant in our country. The unpredictable clinical expression depends on the effect of cofactors such as exercise. The management of avoidance plans can be challenging due to LTP being a widely cross-reacting allergen in plant foods.
Lipid transfer proteins (LTPs) are stable panallergens resistant to heat and pepsin digestion found in many plant foods like fruits, vegetables, cereals, peanut, tree nuts and seeds but also in pollens (plane tree, mugwort, ragweed, wall pellitory, olive and cypress).1,2 They are a common cause of food-induced anaphylaxis (FIA) in adults living in the Mediterranean area.1,3 Furthermore, LTPs have also been proposed as a main cause of food-dependent exercise-induced anaphylaxis (FDEIA).4
LTPs were described as allergens in pollens of mugwort (Art v 3), plane tree (Pla a 3), olive (Ole e 7), wall pellitory (Par j 1-2), ragweed (Amb a 6) and cypress.1,5 These findings led to the hypothesis that food allergy related to LTP might result from a primary sensitization to pollen LTP, which may explain geographical differences in LTP sensitization.6 Studies performed in Southern Europe have reported the relevant role of fruits and vegetables in food allergy, although the allergens involved and the severity of clinical reactions are distinct from those in Northern and Central Europe due to a higher rate of LTP sensitization.6 The peach LTP Pru p 3 seems to be a primary sensitizer. The sensitization route to LTP is still unclear. Along with the oral route, sensitization might also occur through the skin and the airways.1,6
In a Spanish multicenter cross-sectional study including 4991 patients (children and adults) prospectively recruited in outpatient clinics, food allergy was diagnosed in 7.4%. Tree nuts and Rosaceae fruits account for 26% and 24% of the reactions, respectively. Fruits and tree nuts were the most common foods in patients over five years of age. Anaphylaxis was found in 17.9% of patients and exercise-induced anaphylaxis in 2.4%.7
Clinical expression of sensitization is extremely variable, from long-lasting symptomless sensitization to severe anaphylaxis.1 Co-sensitization to labile plant-food allergens (PR-10 family and profilins) might reduce the severity of LTP allergy.8 The presence of cofactors, such as exercise, fasting and non-steroidal anti-inflammatory drugs (NSAID) can amplify the clinical relevance of LTP sensitization.1,9
Diagnosis of food allergy related to LTP sensitization can be particularly challenging considering that the range of plant foods involved is extremely wide. Clinical reactivity to several taxonomically unrelated plant-food groups may raise the suspicion toward LTP sensitization.10 In clinical practice, this is a complex clinical syndrome, due to the spectrum of clinical reactivity. In fact, some patients might react to some plant food and tolerate others that are closely related within the same group.
We aimed to describe the clinical characteristics and the allergen sensitization profiles in patients with FIA related to LTP.
Materials and MethodsAn evaluation was performed of patients with a clinical history of anaphylaxis related to food ingestion followed at our Immunoallergy department. A systematic reporting of anaphylaxis was implemented in our Immunoallergy department over six years (from January 2011 to December 2016). All allergists of the department were invited to participate in the study and a meeting was organized in order to promote the voluntary notification of cases of FIA.
The allergological diagnostic work-up included skin prick tests (SPTs) with multiple plant-food and pollen panel and specific IgE to LTP allergens. LTP sensitization was confirmed either by positive SPT to LTP extract (Pru p 3, Bial-Aristegui®, Bilbao, Spain) and/or in vitro tests (single specific IgE to LTP allergens, ImmunoCAP, or multiplex array, ISAC, Thermo-Fisher®, Waltham, Massachusetts, USA).
Skin prick tests to LTP extract were performed on all patients included in this study, which was positive to all of them, except in a three-year-child who had positive specific IgE to LTP and the mother refused the SPT to her child. In most cases the sensitization was confirmed with both methods. SPT were considered positive if the mean wheel diameter was 3mm or greater than the negative control (0.9% saline) and positive control (histamine 10mg/mL). If no commercial extract or standard specific IgE immunoassay was available, prick–prick tests with the fresh food were made. A cut-off value of ≥0.35kU/L was considered for positivity for single serum-specific IgE and a cut-off value of ≥0.3 ISU (ISAC Standardized Units) was considered for positivity for multiplex array ISAC. All the investigations were performed at least four weeks after the anaphylactic reactions. In case of FDEIA, with clear evidence of specific IgE sensitization to LTP-related foods, patients were successfully challenged with the implicated foods at rest, to confirm their tolerance in the absence of exercise.
ResultsWe report data from 43 patients observed in our Immunoallergy department due to FIA related to LTP, aged 3–52 years, with median age at first allergological evaluation of 29 years, 11 patients (26%) less than 18 years old and 62% female. The median age at first anaphylactic episode was 24 years, ranging from 2 to 51 years. Most patients were atopic (74%), 42% had pollinosis related to LTP sensitization (olive, mugwort, plane tree, wall pellitory and cypress), 22% presented co-sensitization to profilins and only one patient PR-10 sensitization. Besides food allergy, our patients had other allergic diseases: 91% allergic rhinitis, 44% asthma, 7% atopic dermatitis and one patient had eosinophilic esophagitis.
Regarding clinical manifestations, all patients developed mucocutaneous symptoms, 88% respiratory symptoms, 33% had cardiovascular involvement, 16% gastrointestinal complaints and 9% had loss of consciousness. The seven LTP patients also sensitized to profilins and the one patient also sensitized to PR-10 proteins had less severe reactions than the remaining 35 patients (only sensitized to LTP), none of them having loss of consciousness or glottis edema. Despite the severity of the reactions, from the 30 who were evaluated at the emergency department, only six patients were properly treated with epinephrine. Most of the reactions were immediate, 79% occurred within the first 30min after food ingestion, and 31% of patients reported three or more episodes of FIA before the etiological diagnosis had been made. An adrenaline auto-injector was prescribed to all patients, but none needed to use it afterwards.
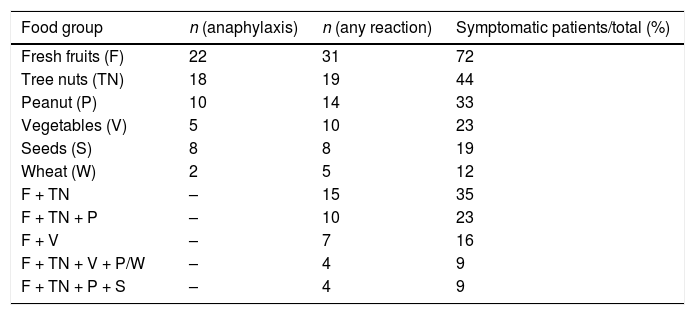
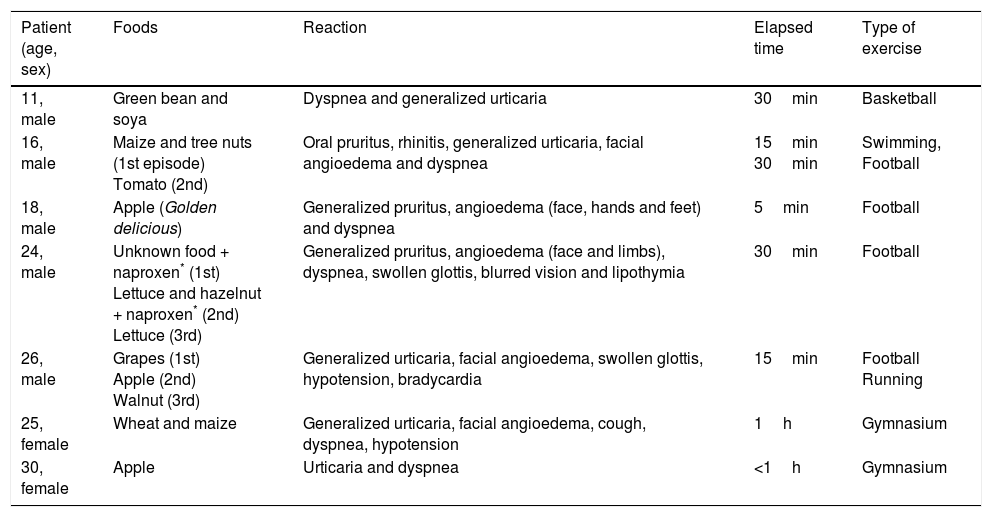
Different food groups were implicated: fresh fruits, including Rosaceae-fruits (peach-11, apple-10 and pear-2) and grape-3 were responsible for 51% of reactions; tree nuts (walnut-6, hazelnut-3, cashew nut-1, mixed nuts-3) accounted for 42%; vegetables including peanut-10, green bean-2, goji berry, tomato, maize, lettuce and wheat represented 40%; seeds (sunflower seed-4 and flaxseed-2) were implicated in 14% (Table 1). In three cases, the culprit food remained unidentified, despite exhaustive investigation. Of interest, we reported seven cases of FDEIA related to LTP in young patients (Table 2). All those patients could tolerate the culprit food at rest. They were advised to avoid exercise for at least four hours after ingestion of the known trigger food and no reactions were reported afterwards. In all other cases of anaphylaxis to LTP we have found the presence of other cofactors clearly implied, namely: fasting in two patients, alcohol in one patient and NSAID intake (acetylsalicylic acid and ibuprofen) in two patients.
Food groups implicated in LTP-allergy symptoms and associations in same patient.
| Food group | n (anaphylaxis) | n (any reaction) | Symptomatic patients/total (%) |
|---|---|---|---|
| Fresh fruits (F) | 22 | 31 | 72 |
| Tree nuts (TN) | 18 | 19 | 44 |
| Peanut (P) | 10 | 14 | 33 |
| Vegetables (V) | 5 | 10 | 23 |
| Seeds (S) | 8 | 8 | 19 |
| Wheat (W) | 2 | 5 | 12 |
| F + TN | – | 15 | 35 |
| F + TN + P | – | 10 | 23 |
| F + V | – | 7 | 16 |
| F + TN + V + P/W | – | 4 | 9 |
| F + TN + P + S | – | 4 | 9 |
Clinical characterization of seven patients with food-dependent exercise-induced anaphylaxis.
| Patient (age, sex) | Foods | Reaction | Elapsed time | Type of exercise |
|---|---|---|---|---|
| 11, male | Green bean and soya | Dyspnea and generalized urticaria | 30min | Basketball |
| 16, male | Maize and tree nuts (1st episode) Tomato (2nd) | Oral pruritus, rhinitis, generalized urticaria, facial angioedema and dyspnea | 15min 30min | Swimming, Football |
| 18, male | Apple (Golden delicious) | Generalized pruritus, angioedema (face, hands and feet) and dyspnea | 5min | Football |
| 24, male | Unknown food + naproxen* (1st) Lettuce and hazelnut + naproxen* (2nd) Lettuce (3rd) | Generalized pruritus, angioedema (face and limbs), dyspnea, swollen glottis, blurred vision and lipothymia | 30min | Football |
| 26, male | Grapes (1st) Apple (2nd) Walnut (3rd) | Generalized urticaria, facial angioedema, swollen glottis, hypotension, bradycardia | 15min | Football Running |
| 25, female | Wheat and maize | Generalized urticaria, facial angioedema, cough, dyspnea, hypotension | 1h | Gymnasium |
| 30, female | Apple | Urticaria and dyspnea | <1h | Gymnasium |
All cases included had positive results of LTP sensitization by means of SPT, except one child whose LTP sensitization was confirmed through specific IgE to Pru p 3. Most of them (81%) had positive results in SPT and specific IgE immunoassay; the remaining patients (7) were tested only with SPT.
The most common foods related to LTP allergens, which were identified through specific IgE immunoassay (single or multiplex array) were: peach (Pru p 3, median value 8.3, n=36), peanut (Ara h 9, median value 3.1, n=21), hazelnut (Cor a 8, median value 2.6, n=18), walnut (Jur g 3, median value 7.7, n=17) and wheat (Tri a 14, median value 4.1, n=3). Among pollens, the most common LTP allergens identified were: mugwort (Art v 3, median value 2.7, n=17), plane tree (Pla a 3, median value 6.9, n=14), wall pellitory (Par j 2, median value 23.3, n=6) and olive (Ole e 7, median value 0.7, n=5). Comparing to SPT, among atopic patients with symptomatic pollinosis (42%), 13 patients were sensitized to olive, 12 to mugwort, 11 to plane tree, eight to wall pellitory and five to cypress.
We found a spectrum of clinical manifestations to different food groups. The same patient often described symptoms with distinct levels of severity from mild-to-severe reactions, depending on the food specimen involved. For instance, some patients developed anaphylaxis with tree nuts but could tolerate Rosaceae fruits. In Table 1, we present all food groups responsible for reactions, regardless of the severity of the reaction, from oral allergy syndrome to systemic reactions, as well as patients who have reacted to more than one group simultaneously. Regardless of the clinical severity, 35% of patients reported symptoms with two different group foods: Rosaceae fruits and tree nuts.
In our department, in the same period, FIA related to LTP sensitization represented 17% of all causes of FIA, from a total of 248 cases of anaphylaxis related to food ingestion.
DiscussionIn our sample, Rosaceae fruits (peach, apple and pear) and grape were the major LTP-related foods responsible for FIA, in more than half cases. Tree nuts (walnut, hazelnut and cashew nut), peanut and other vegetables (green bean, goji berry, tomato, maize, lettuce and wheat) were also important LTP-related foods in FIA, in more than one-third cases, followed by seeds (sunflower seed and flaxseed). Our results are in accordance with published data, in the Mediterranean area, where LTP are major allergens found in Rosaceae fruits, as well as in tree nuts and vegetables.3,10,12 One Spanish study including patients with LTP syndrome (75.6% with anaphylaxis), reported as main offending foods: peach (75.6%), lettuce (48.9%), walnut (46.7%), hazelnut (33.3%), peanut (31.1%) and green bean (26.7%).11
Overall in our center, LTP-induced anaphylaxis represented 17% of all FIA reports. LTP sensitization is linked to severe reactions to foods, as has been proven by other authors.10–12 In our center, among all the causes of FIA, including adult and pediatric patients, LTP sensitization represented the third cause of FIA, after shellfish (20%) and cow's milk (19%), followed by tree nuts (15%), egg (8%), peanut (7%) and fish (4%). A multi-center study in Italian adults reported LTP as the most important allergen causing FIA, and peach was considered the most frequently offending food.13 In our center, LTP was the second cause of FIA in adults (31%), after shellfish (36%).
LTP sensitization might frequently display a broad range of clinical manifestations from mild symptoms to severe reactions with LTP-related foods from botanically unrelated groups. Although our sample is too small to allow conclusions, co-sensitization to labile plant-food allergens (profilins and PR-10 family) could be a preventive factor that might reduce the severity of LTP allergy, as previously described by Pastorello et al.8 Additionally, the presence of co-factors, such as exercise, fasting and NSAID intake, have a strong influence in this variable clinical expression. In our center, LTP-related foods were implicated in 41% of all cases reported with FDEIA (7 out of 17 all cases with FDEIA, from all ages). According to Romano et al.4 LTPs were the most frequent primary allergen (78%) in a group of Italian patients with FDEIA. FDEIA is a rare subtype of anaphylaxis occurring after exposure to an offending food followed by physical activity, while both the implicated food and exercise, isolated, are tolerated independently.14 In some cases NSAID, alcohol, fasting, infections, menstruation, warm or cold temperature or pollen exposure can amplify an exercise-induce effect, and occasionally they are able either to independently substitute exercise or act as an ultimate third co-factor triggering the reaction.14–17 As we found, this disorder is commonest among young adults, however patients of all ages can be affected. Among our cases of FDEIA, we highlight the clinical case of our patient with lettuce allergy who had enhanced reactions due to NSAID intake (naproxen).
Lettuce has been described as a common offending food in patients with LTP syndrome and is associated with a higher risk of severe reactions.18 It is considered an unsuspected food and seldom eaten alone, thus it can be neglected as culprit, delaying the correct diagnosis. Moreover, the presence of cofactors (exercise or NSAID intake) can be essential to elicit the reaction.
Regarding pollen sensitization, there is a relevant association between LTP-induced anaphylaxis and pollinosis in our country. In Portugal, olive and wall pellitory sensitization, along with grass pollen are mainly responsible for pollinosis. According to our experience and data from other countries in Mediterranean area, this higher prevalence of LTP-related food allergy, compared to Northern Europe, might be explained from a distinct pollen sensitization profile. This peculiar type of food allergy is hardly seen in Northern Europe. All reports come from Spain, Italy, Greece and Portugal.9,18–21 Large studies of Rosaceae fruit allergy showed that in Northern and Central Europe there is a predominance of birch pollinosis, thus allergy to apple and cherry seem to be linked to food homologues of Bet v 1, the birch pollen major allergen, with mild-to-moderate reactions. By contrast, in the Mediterranean area, allergy to Rosaceae fruits is related to peach allergy and LTP sensitization, with a higher prevalence of systemic reactions.10,12 This geographical distribution led to the hypothesis of a primary sensitization linked to specific environmental factors present in the Mediterranean area, particularly pollen sensitization and the possibility that the primary sensitization to LTP may occur through airways.1
The management of FIA is centered on avoidance of the culprit food. However, the wide spectrum of symptoms with different food group and the huge list of food containing LTP allergen1 makes strict avoidance particularly difficult to achieve. On the other hand, the food triggers can be inoffensive unless combined with exercise or other type of cofactor, therefore it can be quite difficult to recommend reasonable avoidance. Moreover, we have noticed that some patients began reacting to one food group and later reported symptoms to another food group. Additionally, some patients present mild to moderate symptoms before the development of anaphylactic reactions. According to our experience, this seems to be a growing condition. LTP immunotherapy has shown promising results and clinical effectiveness according to some Portuguese and Spanish studies.22–25
ConclusionsLTP syndrome is a complex clinical pattern due to multiple sensitization to plant foods and pollen. LTPs are important allergens of food-induced anaphylaxis in Portugal. LTP sensitization might be associated with a heterogeneous group of offending plant-foods, taxonomically unrelated, which can induce recurrent reactions. LTP is a useful risk marker in patients with severe reactions due to an unknown cause. Beyond foods related to anaphylactic reactions, patients might report mild to moderate symptoms with other plant-foods, showing that LTP allergy is a complex syndrome.
The clinical expression of sensitization is extremely variable and unpredictable, and might be dependent on the effect of multiple cofactors, such as exercise and NSAID intake. LTPs are widely cross-reacting allergens in plant foods, thus the management of a reasonable avoidance dietary plan can be particularly challenging, including with respect to ingestion before physical activity.
Ethical disclosuresThe authors declare that no experiments were performed for this investigation. The authors declare that they have followed the protocols of their work center on the publication of patient data and that the patients included in the study or their legal guardians have received sufficient information and have given their informed consent in writing to participate in this study.
FundingThe authors declare that no funding was received for the present study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank Thermo-Fisher Scientific® Portugal for the collaboration and support for the realization of the ISAC laboratory tests.