Vernal keratoconjunctivitis is one of the most serious ocular allergies that have the potential to induce large ocular morbidity and significant visual changes affecting the quality of life of these individuals.
MethodologyThis study was conducted in two phases. The first phase consisted of the clinical characterization of 32 patients from the Clinical Allergology center of the I.P.S. Universitaria from July 2014 to February 2015. A retrospective analysis of medical records was performed.
In the second phase, the evaluation of quality of life was conducted using the questionnaire KIDSCREEN 27, which was validated in our population and evaluated as recommended by the creators of this instrument.
ResultsA total of 24 patients (75%) were men, mean age of 12.1 (SD 2.6) years. 100% of the patients had clinical evaluation and were monitored by Allergology and Ophthalmology, 12 patients (37.5%) were found in mild level, 5 patients (15.6%) were moderate and 14 patients (43.8%) were severe level. The most common symptoms were pruritus (75%), photophobia (50%), lacrimation (37.5%), and secretions (28.1%). 65.6% had a family history of atopy and 84.4% had an allergic comorbidity. Aeroallergen skin tests were found positive in 25 patients (78.1%). All patients had initiated ocular treatment by the time the survey of quality of life was conducted; but, they still had low quality of life scores in the 5 domains assessed. When the scores were evaluated by gender, the only statistically significant difference was found in the domain of family life and free time, which was lower for women.
ConclusionThe vernal keratoconjunctivitis is a disease more prevalent in men. It apparently has an important atopic base in our environment, which due to its severe ocular involvement causes a marked decrease in the quality of life of the children who present it.
Vernal keratoconjunctivitis (VKC) is a chronic inflammatory bilateral external eye disease but in its early days can occur unilaterally, affecting mainly patients in the first and second decade of life.1,2 The predominant symptom is severe ocular itching, followed by marked photophobia, blepharospasm, mucus and foreign body sensation.3
It may involve only the palpebral area or only limbo area, but generally occurs in mixed form.4 The hallmark of palpebral VKC is papillary hyperplasia of the upper tarsal conjunctiva, papillae ranging from 1mm in diameter to giant papillae or typical stoned.4 In limbal VKC predominates the infiltration of limbal subconjunctival tissues forming nodules, sometimes accompanied by pannus, superficial neovascularization in cornea's periphery, making limbo's appearance thickened and opaque. They are often covered by white calcified excrescences known as Horner-Trantas nodules.5 The extension to the center of the cornea of limbal vegetations can affect vision,6 also it can be found irregular pigmentation of the bulbar conjunctiva exposed7; all these changes reflecting important ocular involvement of the disease affect the quality of life of patients.
Although some signs and symptoms of VKC may be similar to those of other ocular allergic diseases, the presence of limbo papillae and/or giant tarsal papillae can differentiate it from seasonal allergic conjunctivitis8 and the absence of eczema on eyelids can help to differentiate atopic keratoconjunctivitis.9
It is an often underdiagnosed disease. Although its prevalence varies widely by geographic area, it is considered a rare disease in temperate areas with endemic sites and a prevalence ranging between 4 and 5%10 is estimated. It has the potential to induce serious visual changes of 6–55% of patients, depending on the study population, due to complications of the cornea and unsupervised use of steroids.10 While appropriate assessment of the signs and symptoms of the disease is made with frequency, it is not enough to measure the impact it can have on the daily development of activities in these patients.
Allergic VKC and atopic keratoconjunctivitis (AKC) are the most severe forms of allergic eye disease. Knowing the impact that a disease has on the quality of life, it is important to improve the overall care of the individual, as it helps to evaluate the therapeutic measures and make decisions in health care. Yet, one of the main constraints in this regard are few studies at the time and the difficulty of finding an instrument that is validated to be applied in the target population.
Our study aims to conduct the clinical characterization and evaluate the quality of life of patients with a diagnosis of vernal keratoconjunctivitis, using a scale that is validated in our environment in patients who consulted a reference center of Clinical Allergology of the city of Medellin (Antioquia, Colombia) diagnosed with vernal keratoconjunctivitis.
MethodsA descriptive cross-sectional study was performed; this was carried out in two phases, in the first phase the clinical characterization of patients who came to the Service of Clinical Allergology of the I.P.S. Universitaria was conducted, in a time period from July 2014 to February 2015. A retrospective analysis of medical records was performed, where the following items were evaluated:
- 1.
Patients: Children (age 8–18 years, group for which the application of the instrument of quality of life is allowed) with a diagnosis of VKC. The diagnosis was based on clinical history, previously done by Clinical Allergology and Ophthalmology.
- 2.
Demographic and clinical data: These were taken from the medical records age, gender, place of residence, education, conjunctival papillary reaction, secretion, hyperemia, Trantas nodules, pseudogerontoxon, chemosis, symptoms to determine the activity of the disease at the time to evaluate the quality of life instrument (pruritus and/or photophobia associated with conjunctival hyperemia, discharge, visual disturbances or other clinical signs) and treatments performed.
- 3.
Clinical grades: symptom scale was scored using the proposal described by Bonini et al.11 Subsequently to facilitate statistical analysis, by our sample size, regrouped in mild patients who once Bonini et al. scale was applied, they were in grade 0 (quiescent) and grade 1 (mild intermittent); moderate to those who were in the 2nd grade (intermittent moderate) and grade 2b (moderate persistent) and severe individuals in grades 3 (severe) and 4 (very severe).
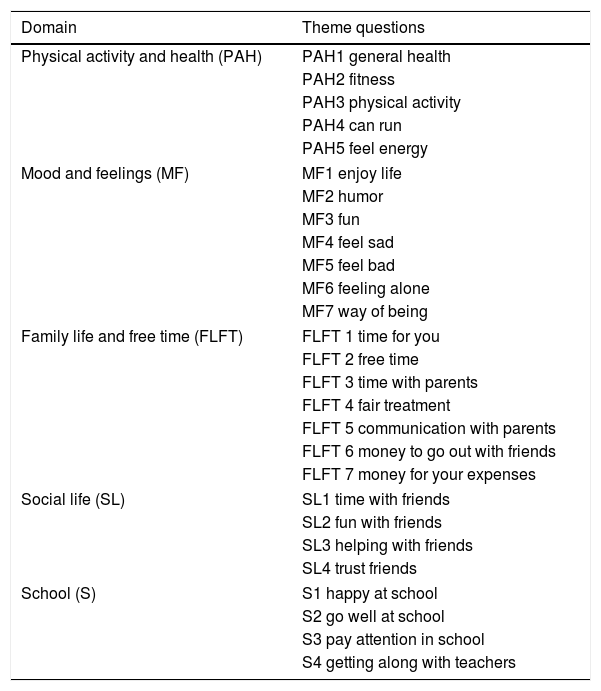
In the second phase the quality of life was assessed. After evaluation of medical records the questionnaire KIDSCREEN 2712,13 to the chosen patients was applied; upon request and approval of its use by the group “The KIDSCREEN Group Europe”. This is a questionnaire consisting of five domains, already validated in our population14; the questionnaire was applied and qualified as indicated in the instructions provided by the authors. The questions that were not answered were imputed with the average value of the items answered in the respective domain, as long as they had responded to 50% of that domain as recommended by the creators of this instrument (Table 1).
Contents KIDSCREEN-27 survey.
| Domain | Theme questions |
|---|---|
| Physical activity and health (PAH) | PAH1 general health |
| PAH2 fitness | |
| PAH3 physical activity | |
| PAH4 can run | |
| PAH5 feel energy | |
| Mood and feelings (MF) | MF1 enjoy life |
| MF2 humor | |
| MF3 fun | |
| MF4 feel sad | |
| MF5 feel bad | |
| MF6 feeling alone | |
| MF7 way of being | |
| Family life and free time (FLFT) | FLFT 1 time for you |
| FLFT 2 free time | |
| FLFT 3 time with parents | |
| FLFT 4 fair treatment | |
| FLFT 5 communication with parents | |
| FLFT 6 money to go out with friends | |
| FLFT 7 money for your expenses | |
| Social life (SL) | SL1 time with friends |
| SL2 fun with friends | |
| SL3 helping with friends | |
| SL4 trust friends | |
| School (S) | S1 happy at school |
| S2 go well at school | |
| S3 pay attention in school | |
| S4 getting along with teachers | |
It was obtained the informed consent from all the patients and the ethics committee of the Universidad de Antioquia and the I.P.S. Universitaria, institutions where the study was conducted, approved the study.
Statistic analysisData were collected in a database in Excel 2013, designed for the study and subsequently exported to SPSS software version 18 for analysis. The Kolmogorov–Smirnov test was used to analyze normality of variables. Those results with normal distribution are described by its mean and standard deviation (SD), for those who did not meet this criterion. The data are presented with median and interquartile range.
Categorical variables are presented as absolute frequencies and percentages. For analyzes, it was determined a confidence level of 95%, an alpha error of 5% and a statistical significance of p<0.05 was determined.
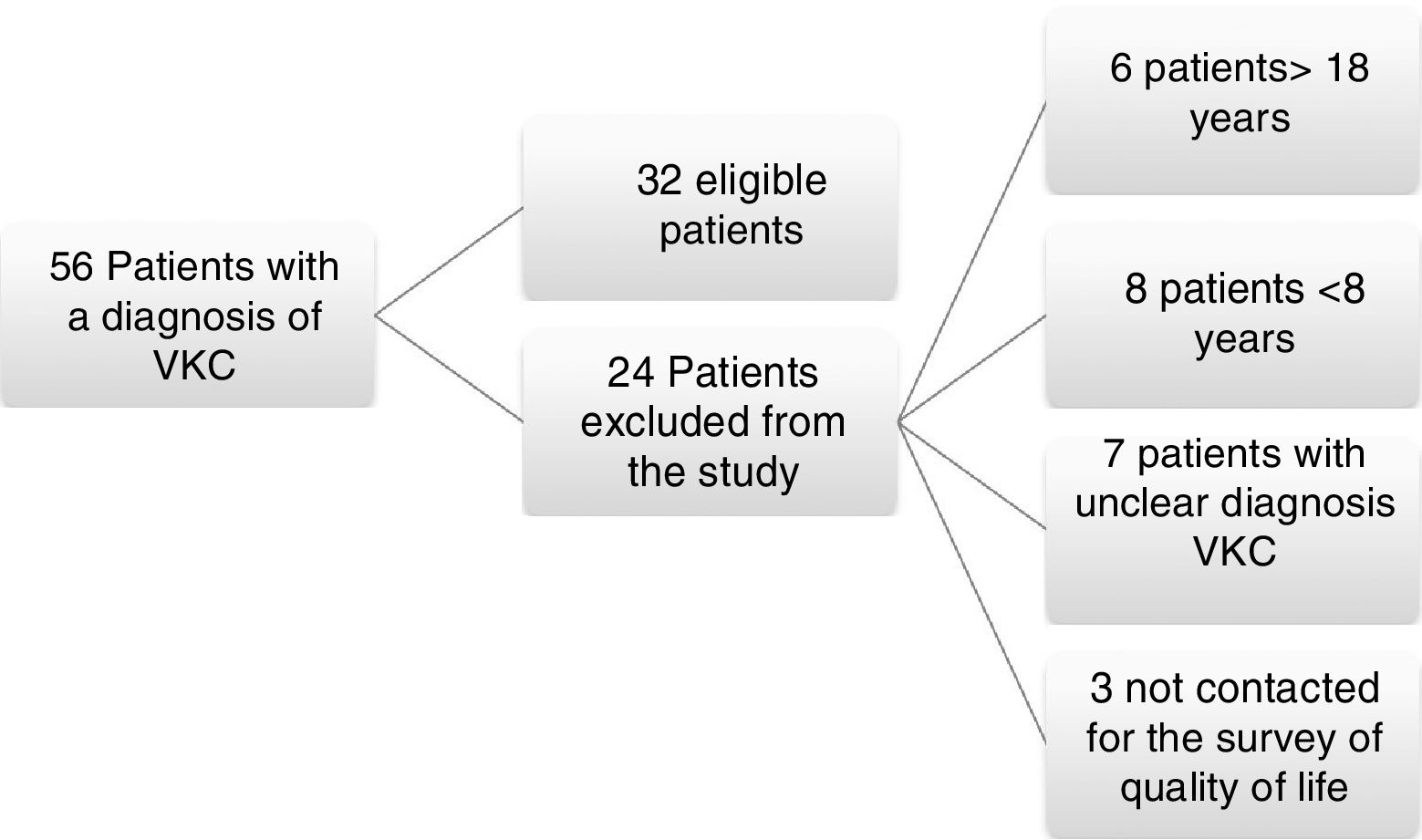
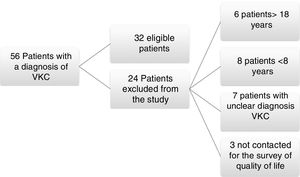
ResultsA total of 56 medical records were found from July 2014 to February 2015 with a diagnosis of vernal keratoconjunctivitis; from these, 24 patients were excluded because of their age, difficulties with medical record in the diagnosis, or for inability to be reached to conduct the survey of quality of life KIDSCREEN 27; 32 patients met the inclusion criteria and were admitted to the study (Fig. 1).
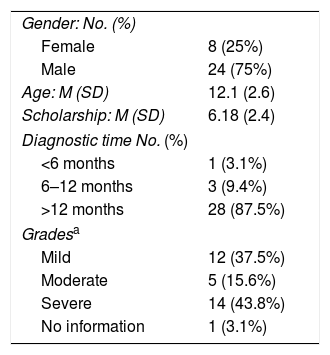
Demographic and clinical characteristics of patients: 24 patients (75%) were men, 79.1% residents in the metropolitan area, with an average age of 12.1 (SD 2.6) years, thirty-two (100%) children were in school, the average approved last year was 6.18 (SD: 2.4). 100% of patients had clinical evaluation and follow-up with Ophthalmology and Allergology, 1 patient with diagnosis made within 6 months, 3 between six months and one year and 28 more than a year. 12 patients (37.5%) were found to be mild grade, 5 patients (15.6%) were in a moderate grade, and 14 patients (43.8%) were in a severe grade (Table 2).
General features and clinics.
| Gender: No. (%) | |
| Female | 8 (25%) |
| Male | 24 (75%) |
| Age: M (SD) | 12.1 (2.6) |
| Scholarship: M (SD) | 6.18 (2.4) |
| Diagnostic time No. (%) | |
| <6 months | 1 (3.1%) |
| 6–12 months | 3 (9.4%) |
| >12 months | 28 (87.5%) |
| Gradesa | |
| Mild | 12 (37.5%) |
| Moderate | 5 (15.6%) |
| Severe | 14 (43.8%) |
| No information | 1 (3.1%) |
The most common symptoms reported by patients when they were in active phase of their disease were pruritus (75%), photophobia (50%), red eye (43.8%), lacrimation (37.5%), discharge (28.1%) and visual disturbances (9%) for a total of 27 symptomatic patients (87.2%); data from one of the patients (3.1%) could not be obtained.
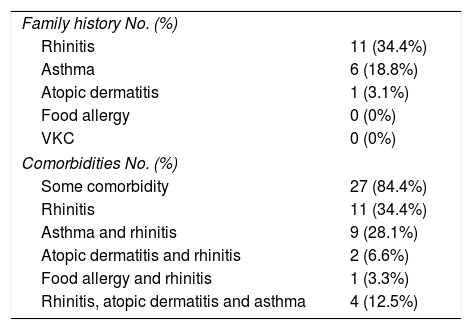
Regarding to the family history of other allergic diseases, it was found that 21 patients (65.6%) reported a first-degree relative with allergic disease. There were no patients with history of a first-degree relative with vernal keratoconjunctivitis or Food Allergy; only one patient reported family history of atopic dermatitis, asthma 6 (18.8%) and the most frequent family history was rhinitis in 11 patients (34.4%) and some patients had various diseases in the family history. Comorbidities such as asthma, rhinitis, atopic dermatitis and food allergy were evaluated. 84.4% of the patients had rhinitis, 11 patients (34.4%) reported rhinitis as the only comorbidity; and, the remaining 50% reported rhinitis accompanied by some other comorbidity with the following distribution: asthma and rhinitis 9 patients (28.1%), rhinitis, atopic dermatitis and asthma 4 patients (12.5%), rhinitis and atopic dermatitis 2 patients (6.6%) and rhinitis and food allergy one patient (3, 3%). 5 patients (15.6%) do not have atopic diseases associated (Table 3).
Family history and comorbidities.
| Family history No. (%) | |
| Rhinitis | 11 (34.4%) |
| Asthma | 6 (18.8%) |
| Atopic dermatitis | 1 (3.1%) |
| Food allergy | 0 (0%) |
| VKC | 0 (0%) |
| Comorbidities No. (%) | |
| Some comorbidity | 27 (84.4%) |
| Rhinitis | 11 (34.4%) |
| Asthma and rhinitis | 9 (28.1%) |
| Atopic dermatitis and rhinitis | 2 (6.6%) |
| Food allergy and rhinitis | 1 (3.3%) |
| Rhinitis, atopic dermatitis and asthma | 4 (12.5%) |
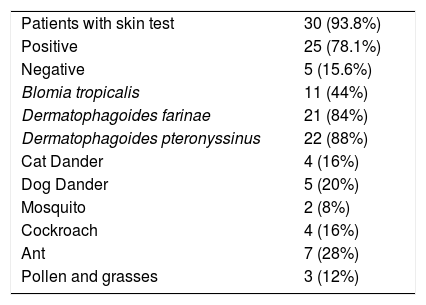
Skin tests for aeroallergens: 30 patients (93.8%) have skin test report aeroallergens, positive in 25 (78.1%) and negative in 5 (15.6%) (Table 4).
Skin tests for aeroallergens.
| Patients with skin test | 30 (93.8%) |
| Positive | 25 (78.1%) |
| Negative | 5 (15.6%) |
| Blomia tropicalis | 11 (44%) |
| Dermatophagoides farinae | 21 (84%) |
| Dermatophagoides pteronyssinus | 22 (88%) |
| Cat Dander | 4 (16%) |
| Dog Dander | 5 (20%) |
| Mosquito | 2 (8%) |
| Cockroach | 4 (16%) |
| Ant | 7 (28%) |
| Pollen and grasses | 3 (12%) |
Sensitization to dust mite 22 patients (88%), for epithelia 6 (24%), insects 9 (36%) and grass pollen and 3 (12%) was found.
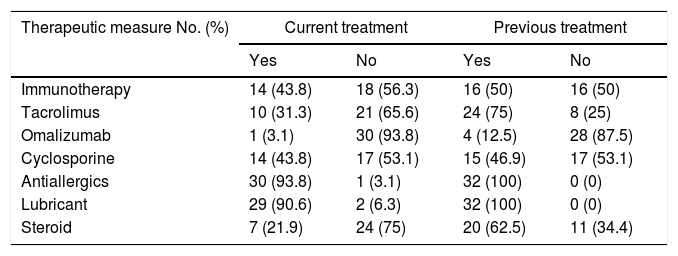
All patients had initiated eye treatment at the time of conducting the survey of quality of life. Information was taken from previous medical record to determine the treatments used to this date. Cyclosporine ophthalmic was received by 14 patients (43.8%) and currently it is used by 15 (46.9%), Tacrolimus was used by 24 individuals (75%) and it continued to be used by 10 patients (31.3%), the Omalizumab was received by 4 patients (12.5%) and only one patient (3.1%) continued to receive it. Regarding the Immunotherapy, it was found that of 25 patients who were sensitized to an allergen, 12 subjects (43.8%) were using this therapeutic measure. Other treatments are detailed in Table 5.
Treatments.
| Therapeutic measure No. (%) | Current treatment | Previous treatment | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Immunotherapy | 14 (43.8) | 18 (56.3) | 16 (50) | 16 (50) |
| Tacrolimus | 10 (31.3) | 21 (65.6) | 24 (75) | 8 (25) |
| Omalizumab | 1 (3.1) | 30 (93.8) | 4 (12.5) | 28 (87.5) |
| Cyclosporine | 14 (43.8) | 17 (53.1) | 15 (46.9) | 17 (53.1) |
| Antiallergics | 30 (93.8) | 1 (3.1) | 32 (100) | 0 (0) |
| Lubricant | 29 (90.6) | 2 (6.3) | 32 (100) | 0 (0) |
| Steroid | 7 (21.9) | 24 (75) | 20 (62.5) | 11 (34.4) |
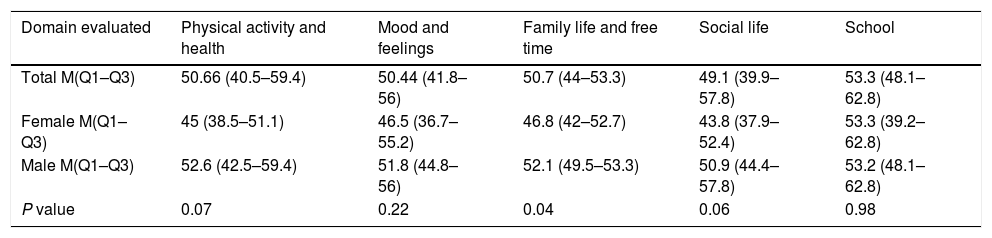
Quality of life: In the 32 patients who were included in the study, quality of life was evaluated by conducting the questionnaire KIDSCREEN 27, 30 patients performed a self-filling out the survey and 2 patients were assisted in reading this. In some domains not all the answers were found and should impute these responses, as recommended by the authors of the scale procedure, these domains were: physical activity and health: In the question “Can run” one patient, domain and Family Life and Free time in the question “amount of money” for one patient and domain School in question “academic performance” two patients. The score by domains for quality of life was determined according to the instructions and equivalence values provided by the authors of the KIDSCREEN 27 group and after applying the score was: physical activity and health (PAH) 50.66 (40.5–59.4), the domain mood and feelings (MF) 50.44 (41.8–56), family life and free time (FLFT) 50.7 (44–53.3), social life (SL) 49.1 (39.9–57.8) and school (S) 53.3 (48.1–62.8). The estimation of each domain is also made by sex and this is shown in Table 6. Finding a lower score in all domains evaluated quality of life except in the Domain School, however this gender difference was only statistically significant for variable Family Life and Free Time.
Quality of life.
| Domain evaluated | Physical activity and health | Mood and feelings | Family life and free time | Social life | School |
|---|---|---|---|---|---|
| Total M(Q1–Q3) | 50.66 (40.5–59.4) | 50.44 (41.8–56) | 50.7 (44–53.3) | 49.1 (39.9–57.8) | 53.3 (48.1–62.8) |
| Female M(Q1–Q3) | 45 (38.5–51.1) | 46.5 (36.7–55.2) | 46.8 (42–52.7) | 43.8 (37.9–52.4) | 53.3 (39.2–62.8) |
| Male M(Q1–Q3) | 52.6 (42.5–59.4) | 51.8 (44.8–56) | 52.1 (49.5–53.3) | 50.9 (44.4–57.8) | 53.2 (48.1–62.8) |
| P value | 0.07 | 0.22 | 0.04 | 0.06 | 0.98 |
Results presented with M: median (Q1–Q3) interquartile range.
There was no statistically significant asocciation among quality life and evaluated parameters as sensitization status, specific allergen immunotherapy, allergic comorbidities and disease severity, but generally all the patients had a lower score of quallity of life in all domains.
DiscussionOur study is the first in Latin America to perform in a cohort of patients with vernal keratoconjunctivitis diagnosis the clinical characterization and measurement of quality of life with an instrument validated in children.
We found that in our series vernal keratoconjunctivitis patients are similar in demographic and clinical characteristics to those reported in the literature at local and global levels.8,15–21 VKC is more common in men, with a male: female ratio of 3:1.
Similar proportions (2.8:1 to 3.3:1) were reported by several authors,8,15,17,21 with the exception of the Ukponmwan study22 who found little difference between genders with a ratio 1.3:1.22 This remarkable difference between genders has suggested that hormonal factors probably play an important role in the development of this disease,23 hypothesis that has not yet been confirmed. Our patients with vernal keratoconjunctivitis refer a high family history of atopic diseases, 65.6%, a finding that is in line with most series reporting frequency ranging from 48.7% to 57.7%,8,15,17,19,21 not reported by Fernandes et al.20; who remarkably found a lower frequency of family history of atopy20 as Leonardi et al. in an adult population,24 both finding a frequency of 28%.20,24 Comorbidity with other allergic diseases has been described with a high frequency15,19 except for Ukponmwan22 who found that only 5% of their patients had any of these diseases as personal history.22 Rhinitis was the most frequent comorbidity (84%) that was always present in subjects who showed some pathological personal history, followed by asthma (40.6%) and atopic dermatitis (18.8%). Trends confirmed in the literature.15,17,20 The presence of specific IgE measured by skin test is reported as important in our population,25 had already been described in VKC by Ramirez et al. (88%)15 and it is now reiterated by our study, 78%, numbers according to the reported in a cohort of Brazil by De Oliveira et al.,21 and in front of the two European courts, reported by Bonini et al. (57.8%),8 and Leonardi et al. (43.7%).17 Suggesting that perhaps there is greater sensitization to some allergen in the tropics or greater sensitization of these for be perennial.
The main symptom manifested by individuals affected by this disease is itching, followed by photophobia and secretions; these clinical symptoms were reported in all series as the most frequent.8,15,17,19–22
Treatments used in vernal keratoconjunctivitis subjects were generally similar to those used traditionally for this disease8,15,17; although, in our study we found using other molecules in moderate and severe cases, such as Tacrolimus and Omalizumab. Immunotherapy also appeared as a frequent alternative in patients sensitized to aeroallergens; treatment that was also described as effective therapeutic measure in our population affected by VKC by the group Experimental Clinical Allergology of the Universidad de Antioquia.26
Vernal keratoconjunctivitis is one of the most severe forms of ocular allergy, it is not uncommon to think that the quality of life of individuals who have this disease will be affected. It is believed that the measurements of the quality of life through scales may be useful to the health personnel, not only to understand the impact of the disease on the activities of daily living of individuals but also to determine the effect of treatments they use helping to take clinical decisions. This is the first study measuring quality of life in patients with vernal keratoconjunctivitis diagnosis conducted in Latin America, with a validated scale and in Medellin, Colombia. We use a generic questionnaire for quality of life because although Sacchetti et al.27 developed a questionnaire to measure quality of life in pediatric population diagnosed with vernal keratoconjunctivitis, it could not be used in our population because it was not even validated in our environment.
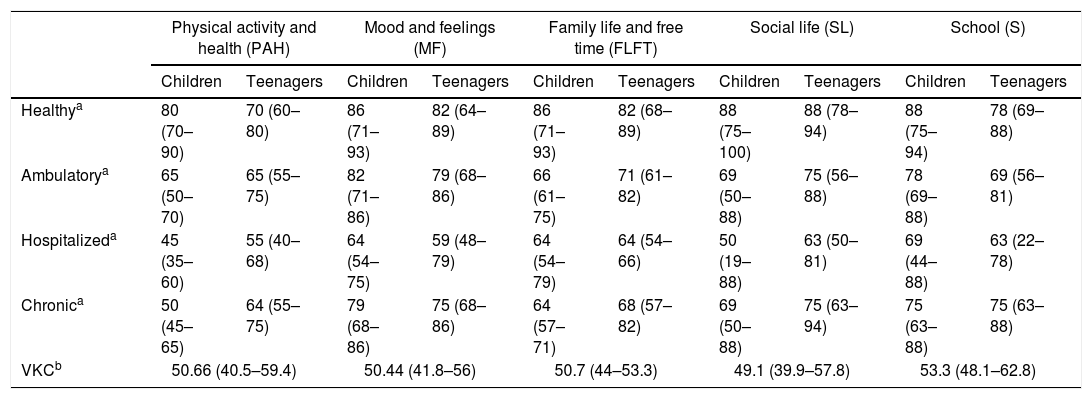
We found that the quality of life of children evaluated was compromised in the different domains assessed, this evaluated more objectively when compared even with the results obtained in the validation study of this scale in our city, scale that was validated in 321 children, 160 with disease and 161 healthy14 (between 8 and 18 years old, the same age range that our study), where scores are at lower levels for all areas against the population of the same healthy age, as detailed in Table 7. It can be further noted that the scores in most domains of individuals affected by VKC are even lower than those qualified by children with chronic illnesses, giving very similar results to the population of validation cohort questionnaire that was hospitalized14 (Table 7).
Quality of life in VKC compared with the Instrument Validation study KIDSCREEN-27.
| Physical activity and health (PAH) | Mood and feelings (MF) | Family life and free time (FLFT) | Social life (SL) | School (S) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children | Teenagers | Children | Teenagers | Children | Teenagers | Children | Teenagers | Children | Teenagers | |
| Healthya | 80 (70–90) | 70 (60–80) | 86 (71–93) | 82 (64–89) | 86 (71–93) | 82 (68–89) | 88 (75–100) | 88 (78–94) | 88 (75–94) | 78 (69–88) |
| Ambulatorya | 65 (50–70) | 65 (55–75) | 82 (71–86) | 79 (68–86) | 66 (61–75) | 71 (61–82) | 69 (50–88) | 75 (56–88) | 78 (69–88) | 69 (56–81) |
| Hospitalizeda | 45 (35–60) | 55 (40–68) | 64 (54–75) | 59 (48–79) | 64 (54–79) | 64 (54–66) | 50 (19–88) | 63 (50–81) | 69 (44–88) | 63 (22–78) |
| Chronica | 50 (45–65) | 64 (55–75) | 79 (68–86) | 75 (68–86) | 64 (57–71) | 68 (57–82) | 69 (50–88) | 75 (63–94) | 75 (63–88) | 75 (63–88) |
| VKCb | 50.66 (40.5–59.4) | 50.44 (41.8–56) | 50.7 (44–53.3) | 49.1 (39.9–57.8) | 53.3 (48.1–62.8) | |||||
The same scale was used to evaluate the impact of immunotherapy in the quality of life of patients with rhinitis.28 Individuals diagnosed with rhinitis prior to performing immunotherapy had similar results in the various domains, except for the domain psychological wellbeing having a lower score in patients with rhinitis; these results suggest that as rhinitis, vernal keratoconjunctivitis is a disease that has an important impact on the quality of life of patients.
We found a study of quality of life in adult patients with vernal keratoconjunctivitis, which concluded that decreased productivity and social activities was not considered a limiting factor.9 No association was found against the grade of disease and quality of life; only a trend is shown that could be explained by the size of the sample, which when divided by groups does not represent an adequate number for the analysis.
In our study, no statistically significant difference was found between the quality of life of patients and different clinical variables with which this was crossed as expected. The majority of patients for example, were in a severe grade of the disease (43.8%%), which involved comparing a robust endpoint to a few data, this maybe due to the size of the sample. This was reduced for several reasons that include the lack of specific questionnaires for the disease to allow cover a wider age population without having to leave out those under 8 years old, who represent an important group given the age of onset of the disease; the severity found in our population, hypotheses that speak of the need for more studies that permits subgroup analysis allowing greater approach to the individual needs of patients and making it possible to answer such questions.
ConclusionVernal keratoconjunctivitis in our population is a serious eye disease that has the same epidemiological characteristics already described in the literature; with significant comorbidity with other allergic diseases and sensitization clinically relevant to aeroallergens leading to think that apparently has an important atopic base, which by its potential severe eye complications, has a major impact on the quality of life of children who present it.
FundingNo external funding sources were used for carrying out this study.
Conflict of interestsThe authors declared no conflicts of interest.
The authors thank Dr. Ana Victoria Valencia Duarte. Teachers, Residents and Nursing Staff, Program Clinical Allergology. Universidad de Antioquia.