Several application routes as subcutaneous (SCIT), sublingual (SLIT), intranodal (ILIT), oral (OIT) and rectal delivery routes have been applied for allergen-specific immunotherapy (AIT) so far. However, certain delivery routes likewise rectal, intralymphatic and oral application are less established in routine practice or can be accompanied by severe adverse events. Due to the restricted volume intracutaneous application of allergen is only applicable for diagnostic purpose rather than for intervention studies. Whether novel application routes (e.g. OIT and ILIT) lead to a sustained, ‘robust’ development of tolerance in patients has not yet been thoroughly investigated. Utilization of novel delivery routes therefore is still a challenge to improve safety and efficacy of AIT. In line with this EPIT has become of particular interest in the past few years.
Epicutaneous immunotherapy (EPIT) using patches aims to develop sustained sensitization or tolerance by continuous (and constant) allergen exposure to the skin. Several advantages have been proposed for EPIT: It (1) provides a high safety profile due to an allergen application into the non-vascularized epidermis and subsequently allergen delivery to the less vascularized dermis, (2) increases the convenience for the patients by a non-invasive (needle-free) and self-administrable application method, likely leading to an improved compliance, (3) is free of additional potential irritant constituents (e.g. alum, or preservatives), and (4) will be less cost intensive than conventional AIT.
EPIT intends to target skin-resident immune cells. Allergens delivered to the epidermis are taken up by (a) epidermal Langerhans cells (LC), which become activated and migrate into the dermis or (b) diffuse into the dermal compartment where they can be captured by dermal dendritic cells (dDC). Subsequently antigen presenting cells (APCs) migrate to skin-draining lymph nodes where they can activate the adaptive immune system to induce T cell polarization and tolerance. So far it is remains unclear whether and to what extent APCs directly can activate dermal resident T cells. The epidermis (0.05–0.15mm thickness) consists of a lipid-rich matrix and tight junctions and acts as a physiological barrier, which is usually impermeable for molecules larger than 500Da. Therefore, microneedles, adhesive tape stripping or abrasion of the skin (by using a foot file) have been applied in clinical studies to increase the permeability of the skin. By contrast, in other studies a hydration facilitated allergen delivery without previous pretreatment of the skin has been used (Viaskin® patch technology, DBV, Montrouge, France). Viaskin® patches are generated by using an electrospray to attach a liquid solution of electrically charged proteins onto the patch's backing, which is then turned into a dry and homogenous protein layer, which remains stuck onto the patch's backing [www.dbv-technologies.com]. Once applied on intact skin, the patch forms a condensation chamber, which hydrates the skin and solubilizes the antigen allowing it to penetrate into the epidermis. The approach suggests that mechanical or chemical pre-treatment of the skin to increase the permeability can be circumvented by maceration of the skin. Of note any type of forcible barrier disruption triggers an immune response, e.g. the activation of epidermal keratinocytes. Keratinocytes have been suggested (1) to induce LC-migration into the dermis by secretion of G-MCF and IL-34, (2) to release pro-inflammatory cytokines (IL-1β, IL-6, IL-18 and TNF) which in turn activate dermal DCs in the presence or absence of antigens, and (3) to promote TH2-licensing of dendritic cells. Actually the effect of the degree of skin pre-treatment and barrier disruption on the activation of dermal immune cells (dDC, macrophages, T cells, innate lymphoid cells, and mast cells), and the subsequent consequence for the safety and efficacy of EPIT remains to be further investigated.
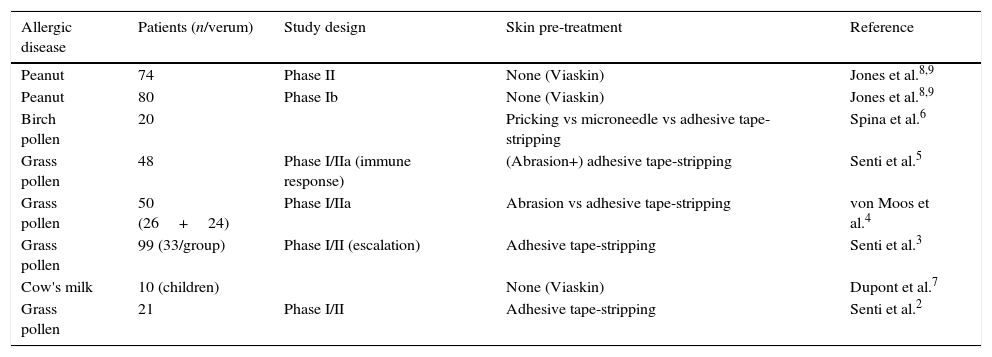
Clinical development of EPITFirst successful EPIT has been applied almost one century ago. Vallery-Radot and colleagues (1921) treated a horse hair-allergic patient by EPIT on scarified skin. Later EPIT was applied for treatment of pollen allergy. The first time the epidermis was suggested as immunologic organ might be more suitable for AIT than the subcutaneous tissue [reviewed in Ref. 1]. The revival and systematic investigation of EPIT started in 2009/2010, mainly by Senti and Kündig to prevent of pollen allergy, and Dupont and Benhamou to prevent food allergy. Remarkably only a limited number of clinical studies had been published so far (Table 1). The clinical studies were performed by using allergen extracts (from grass & birch pollen, or peanut) and cow's milk allergens for the intervention of pollen and food allergy. All clinical studies were performed without additional adjuvants.
Clinical studies of epicutaneous immunotherapy (peer-reviewed publications).
| Allergic disease | Patients (n/verum) | Study design | Skin pre-treatment | Reference |
|---|---|---|---|---|
| Peanut | 74 | Phase II | None (Viaskin) | Jones et al.8,9 |
| Peanut | 80 | Phase Ib | None (Viaskin) | Jones et al.8,9 |
| Birch pollen | 20 | Pricking vs microneedle vs adhesive tape-stripping | Spina et al.6 | |
| Grass pollen | 48 | Phase I/IIa (immune response) | (Abrasion+) adhesive tape-stripping | Senti et al.5 |
| Grass pollen | 50 (26+24) | Phase I/IIa | Abrasion vs adhesive tape-stripping | von Moos et al.4 |
| Grass pollen | 99 (33/group) | Phase I/II (escalation) | Adhesive tape-stripping | Senti et al.3 |
| Cow's milk | 10 (children) | None (Viaskin) | Dupont et al.7 | |
| Grass pollen | 21 | Phase I/II | Adhesive tape-stripping | Senti et al.2 |
Senti and Kündig performed EPIT with adhesive tape stripping,2,3 or a combination of abrasion or microneedles and adhesive tape stripping4–6 to increase the permeability of the skin. The authors observed no serious adverse events by EPIT upon tape-stripping, although higher allergen doses were associated with drug-related adverse events.2,3 Local immediate-type reaction and TEWL were less pronounced by tape-stripping than by abrasion.4 In addition, systemic adverse reactions were more frequent after EPIT and abrasion (6/26) than after tape-stripping (1/24). Of note, the design of the three studies varied by source and potency of the allergen extract, solvent, frequency of tape-stripping, number of patches and consequently the cumulative allergen dose, as well as the exposure time of the patches. Nevertheless all studies demonstrated efficacy of EPIT which was attributed to a moderate increase of allergen-specific IgG4 response.5 The authors concluded that treatment efficacy was determined by the allergen dose, whereas local side-effects depend on the administration time of the patches and systemic reaction were related to the degree of the skin barrier disruption.5 Finally, microneedle (<100μm) pre-treatment induces stronger immediate-phase reactions (100% of patients) than tape-stripping (15% of patients), at which 3/20 patients develop eczema.6 The authors concluded that microneedle treatment enhances the skin barrier disruption and subsequent allergen penetration, and is a promising method for EPIT due to the induction of DC-mediated T cell response.
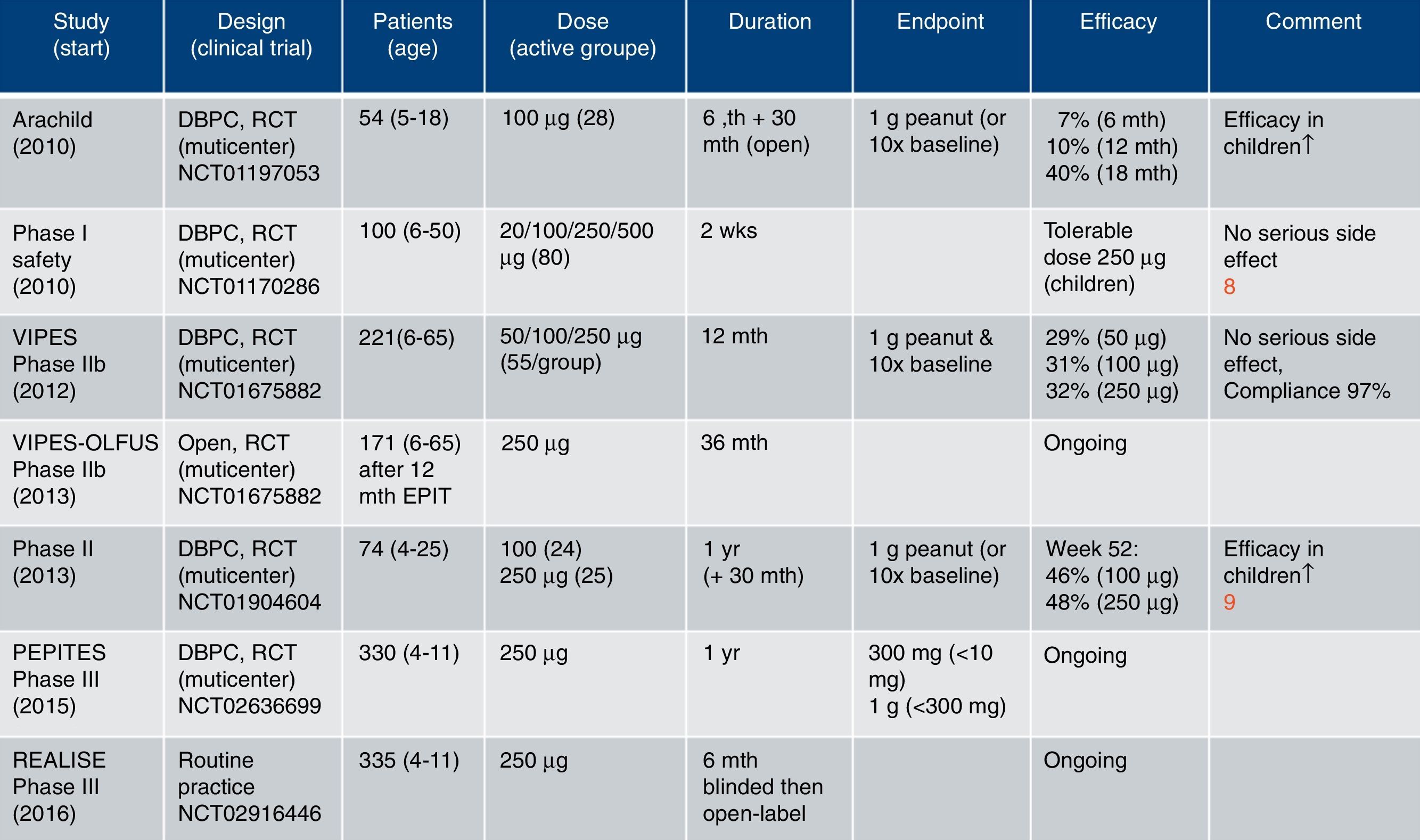
In contrast, allergen delivery through the intact (hydrated) skin is an alternative strategy applied by the Viaskin® technology. The technology preferentially has been developed for the treatment of food allergies, likewise egg (preclinical stage), cow's milk (Viaskin® Milk, phase II)7 and peanut (Viaskin® Peanut, phase III)8,9 [www.dbv-technologies.com]. Although several clinical trials (all including children) for the treatment of peanut allergy have been initiated since 2010, currently only a limited number of studies have been published recently in peer-reviewed journals (Fig. 1).
Clinical studies using Viaskin® Peanut for EPIT [www.dbv-technologies.com].15
The results of a multicenter, double-blind, randomized, placebo-controlled phase II study including 74 peanut allergic children and adults have been reported recently.9 Upon and increasing exposure time until week 22 patients were treated with peanut patches (Viaskin® Peanut 100μg and 250μg) over 52 weeks. Primary endpoint for successful treatment was defined as at least 10-fold increase (in comparison to baseline) of the threshold to elicit clinical symptoms, and more restrictive the tolerance of 1g peanut protein. Successful treatment was achieved in 46% and 48% of the verum groups, respectively. The study confirmed previous reports that the beneficial effect was higher in children (<11 years, 59–66%) than in adolescents and young adults (>11 years). Nearly 80% of treated patients developed local mild reactions, whereas the compliance was around 97%.
In summary, EPIT, both after and without pre-treatment of the intact skin is a promising strategy for the prevention of allergies. EPIT frequently induces local mild reactions but no adverse systemic reactions and can be considered as safe.
Evaluation of EPIT using recombinant Bet v 1 and an adjuvant in an experimental mouse model of birch pollen allergyLike other AIT, EPIT aims at inducing desensitization or clinical tolerance, which could be achieved by inducing TH1 cells and/or regulatory immune cells producing immune-suppressive cytokines, and/or generating blocking antibodies to antagonize binding of IgE to the allergens.
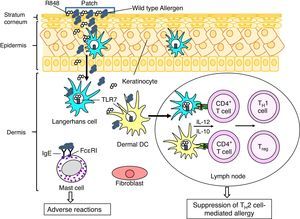
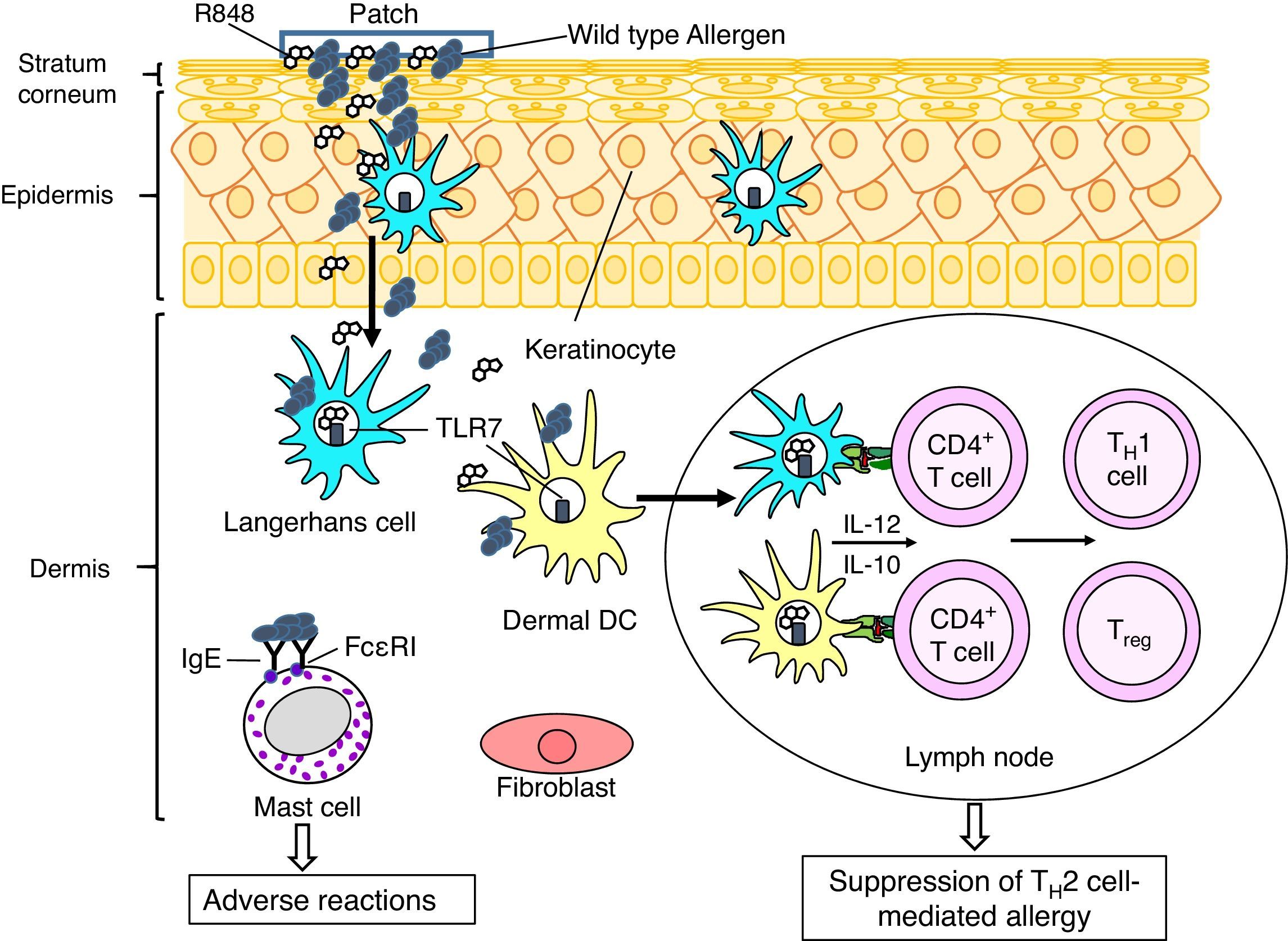
In this context we performed preclinical studies to investigate whether recombinant allergens (using the major birch pollen allergen Bet v 1 as model) can be utilized for EPIT, and whether the desired beneficial effect can be strengthened by co-administration of adjuvants. Adjuvants recognized by Toll-like receptors (TLRs) could be applied to promote the counter-regulation of TH2 responses by inducing TH1-type and/or regulatory cytokines by DCs, T cells and/or B cells. The adjuvant effects of TLR agonists are different depending on their receptors and application routes, since compositions of immune and structural cells expressing TLRs are different among tissues. We demonstrated that a TLR7 ligand R848 is a promising adjuvant in EPIT to treat allergic asthma using a murine model of birch pollen allergy.10 When compared to EPIT with recombinant Bet v 1 (rBet v 1), EPIT with rBet v 1 plus R484 significantly suppressed airway eosinophilia and hyperreactivity in asthmatic mice. LCs and dDCs express TLR7 in their endosomes.11 Targeting TLR7 R848 could induce TH1- and/or regulatory T cells by stimulating LCs and dDCs to produce IL-12, a cytokine involved in differentiation of TH1 cells, and/or IL-10, a regulatory cytokine, and thereby suppress asthmatic features in EPIT (Fig. 2).
A putative therapeutic mechanism in EPIT to treat allergy. Allergens, which are applied on the surface of skin using a patch, are captured by Langerhans cells (LCs) in the epidermis and dermal dendritic cells (dDCs). These antigen presenting cells move to lymph nodes to prime CD4+ T cells. LCs, DCs, keratinocytes and fibroblasts in cutaneous tissues produce pro-inflammatory and anti-inflammatory cytokines, depending on stimuli. Use of an adjuvant, e.g. a Toll-like receptor 7 ligand R848, inducing TH1 cytokines (e.g. IL-12) and/or regulatory cytokines (e.g. IL-10) in LCs and DCs would promote induction of allergen-specific TH1 cells and/or regulatory T cells (Treg), which lead to suppression of TH2 cell-mediated allergy. It should be noticed that mast cells also present in dermis. EPIT using wild type allergens has a risk of adverse reaction due to interaction of the allergens with IgE antibodies captured by Fc¿RI on the cell surface of mast cells. To reduce the risk of adverse reaction, use of hypoallergenic derivatives has been considered.
In order to improve the efficacy and safety of EPIT, we also assessed a use of a hypoallergenic variant with reduced IgE antibody binding capacity as a therapeutic allergen in the epicutaneous treatment. EPIT using wild-type allergens carries a risk of adverse reactions through activation of dermal mast cells via Fc¿RI engagement (Fig. 2), since allergens in their native 3-dimensinal structure usually present conformational IgE-reactive epitopes. Due to reduced allergic potency, hypoallergenic derivatives have been suggested as safer and potentially more efficacious alternative to the corresponding wild-type allergens in AIT. We previously found that Bet v 1B2, a mutant generated by side-directed mutagenesis, displayed remarkably lower IgE binding in almost all tested sera of birch allergic patients.12 Bet v 1B2 possesses a substitution of serine at position 112, which reduces IgE binding of Bet v 1B2 due to the disruption of protein conformation.13 Notably, EPIT with rBet v 1B2 but not with rBet v 1 suppressed development of allergic features in a murine model of birch pollen allergy.14 Lysosomal digestion assay suggested that rBet v 1B2 is processed more efficiently to generate T cell epitope peptides than rBet v 1 in antigen presenting cells. It suggests that EPIT with rBet v 1B2 induces higher levels of Bet v 1-specific TH1 cells and/or regulatory T cells than rBet v 1, and therefore suppresses TH2 cell-mediated allergic asthma.
In summary, we demonstrated that use of a TLR7 ligand, or a hypoallergenic variant of Bet v 1 improves the efficacy of EPIT to treat birch pollen induced allergic asthma. Hypoallergenic derivatives likely can be applied in higher doses, due to their reduced allergenicity, and therefore provide a safer and more effective treatment than wild type allergens. It would be important to assess whether use of a hypoallergenic variant in combination with a proper adjuvant inducing TH1 cells and/or regulatory cells further promote the efficacy of EPIT.
Summary and outlookEpicutaneous immunotherapy (EPIT) is a developing technique with a great potential for an efficient tolerance induction in food and inhalant allergies along with a reduced risk of adverse side effects. EPIT is needle-free and self-administrable, and therefore highly convenient and attractive for the patients. Although EPIT conducted in controlled clinical studies showed a high compliance, it should be considered that this might not be the case by “non-controlled” self-administrable application by the patients themselves. Currently the results of only a very limited number of clinical studies have been published. In line with this, long term effects by EPIT in regard of a sustained tolerance induction have not been described so far. Despite the immune mechanism of EPIT in mice is well investigated the mechanism of desensitization and the skin immune cells involved remains to be elucidated in patients. EPIT can be performed with or without previous pre-treatment of the skin. Pre-treatment of the skin enhances the permeability which facilitates allergen delivery to the immune cells and finally triggers the skin immune response (keratinocyte activation and T cell polarization). Of note, the degree of skin-barrier disruption seems crucial for both safety and efficacy. It seems questionable how the pre-treatment procedure, in particular tape-stripping, can be standardized. Other factors likely influencing the permeation of allergens (e.g. by compromised skin integrity, inflammatory disease, hydration, pH, and skin temperature, as well as the position of patch application) are less investigated. Efficacy of EPIT (using Viaskin® technology) has been primarily demonstrated for children. In line with this it remains to be investigated whether a higher dose of allergen would provide further benefit also for adult patients. Certainly, several parameter need to be modified during the clinical development of the EPIT. Consequently the comparability between the studies is limited due to a different study design. Further studies are necessary to optimize the treatment regime (by means of the pre-treatment modalities, allergen dose, number and exposure time of patches, interval between patch application, etc.) in order to balance between safety and efficacy. These clinical studies are currently ongoing and will provide further data within the next few years.
Along with the clinical development of EPIT efforts to further improve the treatment are currently running. Whereas clinical studies are performed with allergen extracts we and others showed the applicability of purified recombinant allergens for EPIT in experimental allergy models in mice. Moreover we demonstrated that co-application of R848, a TLR7 ligand, or a hypoallergenic variant of Bet v 1 improves the efficacy of EPIT in a mouse model of birch pollen induced allergic asthma. It would be interesting to prove whether these approaches can be transferred to clinical application in future.
Conflict of interestDr. Scheurer reports grants from German Research Foundation, grants from Federal Ministry of Education and Research, outside the submitted work.
Dr. Toda has nothing to disclose.




![Clinical studies using Viaskin® Peanut for EPIT [www.dbv-technologies.com].15 Clinical studies using Viaskin® Peanut for EPIT [www.dbv-technologies.com].15](https://static.elsevier.es/multimedia/03010546/00000045000000S1/v1_201711201851/S0301054617301325/v1_201711201851/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)