Atopic dermatitis (AD) is a multifaceted disease that involves a complex interplay between the skin and the immune system. The course of the disease depends strongly on the genetic background of the patient and on yet poorly-defined environmental factors. Changes in lifestyle could be behind the dramatic rise in the prevalence of AD across continents; including hygienic conditions, food, social habits, skin microbiome or exposure to a number of allergens. Although AD typically develops in childhood and disappears after a few years, in a relatively large number of patients it continues into adulthood. Adult AD can also appear de novo but it is often underdiagnosed and its treatment can be challenging. New, highly effective drugs are being developed to manage moderate and severe forms of the disease in adults. In this review, we highlight the most recent developments in diagnostic tools, current insights into the mechanistic basis of this disease, and therapeutic innovations.
Atopic dermatitis (AD, atopic eczema) is a chronic, inflammatory, and intensely pruritic skin disease.1,2 It is the most common cutaneous disease in children. AD is characterised by a hyperactive immune response to environmental factors and dry, itchy skin. Skin lesions can cause considerable psychological distress and a dramatic burden in the quality of life of patients and their families. AD is characterised for its fluctuations, its potential reversibility, and unpredictable progression in the life of the patient. Skin lesions can be triggered by stress, by contact allergens, and by scratching, among other factors. Onset of the disease in different periods of childhood has distinct outcomes later on and, although most cases resolve themselves in adolescence, a fraction of childhood patients develop the disease in adulthood.3 Yet, AD can also develop de novo in adults or even at an advanced age.
Although the clinical manifestations of the disease can be surprisingly uniform, it is now known that AD presents a great degree of underlying heterogeneity. This heterogeneity stems from the variety and complexity of the mechanisms of pathogenicity of AD.4 AD is a multifaceted disease resulting from the interaction network of the skin components (cellular and extracellular components contributing to the skin barrier), the immune system (innate and adaptive), and the skin microbiome.1 The multiplicity of pathogenic factors has led to discussion of what components are more critical for triggering the disease, or which should be the target of therapies.
AD often occurs in families with atopic diseases, reflecting a strong genetic component in its pathogenesis. Still, the development and progression of AD can be greatly influenced by environmental factors. A large number of epidemiological studies have pointed towards diverse causes, many of which seem to have issues in common with the early development of the immune system and with triggers of chronic inflammation.5
AD can be a challenging disease to treat, especially in its more severe forms.6 However, a number of novel therapies have emerged in recent years. Especially relevant are biologics targeting the immune system and in particular interleukins relevant to atopic diseases. These drugs hold especial promise for adult patients with moderate or severe forms the disease.7,8
Diagnosis of AD relies on clinical signs, allergy tests, and exclusion of other skin diseasesDiagnosis of AD is always made following clinical criteria and generally does not require complementary tests. The clinical presentation of AD depends on the age of the patients and the stage (acute or chronic) of the disease.2,9 The criteria first defined by Hanifin and Rajka have been used for many years in the clinical diagnosis of AD,10 and later also those of the UK working party.11 Both systems are based mainly in the identification of atopic signs in the skin (especially skin folds) and time of appearance of the disease, among a number of other factors (Table 1). The diagnostic criteria listed can be found in most AD patients and should be sufficient to reach a definite diagnosis, especially in adults. Other skin conditions must be excluded when diagnosing AD, and often this requires complementary testing in the form of patch testing, prick tests or biopsy. Patch-testing for contact allergens is advisable in children and adults with moderate-to-severe AD, especially those with Eczema Area and Severity Index (EASI) scores greater than 10. It also should also be performed on adult patients refractory to treatments to exclude possible contact allergy sensitisation, or to those with a de novo skin eczema.1,2,12,13 Patients with hand or foot dermatitis should always be patch-tested.14 Prick tests should be aimed at detecting food allergies (mostly in children) or sensitisation to aeroallergens in adults with severe AD. Skin biopsy is unspecific as it shares findings with other eczemas, but can be considered as a confirmation of AD and to exclude other conditions such as dermatitis herpetiformis, early-stage cutaneous T-cell lymphoma, palmoplantar psoriasis, childhood scabies, or erythroderma, among others.
Diagnostic criteria for AD.
| Hanifin and Rajka | |
| Major criteria (any three of the following) | • Pruritus • Dermatitis affecting flexural surfaces in adults or face and extensor surfaces in infants • Chronic or relapsing dermatitis • Personal or family history of cutaneous or respiratory allergy |
| Minor criteria (any three of the following) | • Facial features (facial pallor, erythema, hypopigmented patches, infraorbital darkening, cheilitis, infraorbital folds, recurrent conjunctivitis, anterior neck folds) • Triggers (emotional factors, environmental factors, food, skin irritants) • Complications (susceptibility to skin infections, impaired cell-mediated immunity, predisposition to keratoconus and anterior subcapsular cataracts, immediate skin reactivity) • Other (early age of onset, dry skin, ichthyosis, hyperlinear palms, keratosis pilaris, hand and foot dermatitis, nipple eczema, white dermatographism, perifollicular accentuation) |
| UK Working Party | |
| In addition to itchy skin, any three of the following: | • Visible flexural eczema (e.g., antecubital and popliteal fossae) or visible dermatitis of the cheeks and extensor surfaces if under 18 months • Personal history of dermatitis • Personal history of dry skin in the last 12 months • Personal history of asthma or allergic rhinitis (or history of eczema in a first degree relative if <4 years old) • Onset of signs and symptoms under the age of 2 years (this criterion should not be used in children <4 years) |
| American Academy of Dermatology | |
| Essential features (must be present) | • Pruritus • Eczema (acute, subacute, chronic) ■ Chronic or relapsing history ■ Typical morphology and age-specific patterns: - Facial, neck, and extensor involvement in infants and children - Current or previous flexural lesions at any age - Sparing of the groin and axillary regions |
| Important features (seen in most cases, adding support to the diagnosis) | • Early age of onset • Atopy (personal and/or family history; raised IgE levels) • Xerosis |
| Associated features (suggestive of AD but too non-specific to be used for defining or detecting AD for research studies) | • Atypical vascular responses (e.g., facial pallor, white dermatographism, delayed blanch response) • Keratosis pilaris, pityriasis alba, hyperlinear palms, ichthyosis • Ocular, periorbital changes • Other regional findings (e.g., perioral, periauricular lesions) • Perifollicular accentuation, lichenification, prurigo lesions |
| Exclusionary conditions | Scabies, seborrheic dermatitis, contact dermatitis (irritant or allergic), ichthyoses, cutaneous T-cell lymphoma, psoriasis, photosensitivity dermatoses, immune deficiency diseases, erythroderma, etc. |
Currently there are no validated biomarkers that can readily help in the diagnosis of AD, although it is expected that some will emerge in upcoming years.15 Although it has been estimated that about 80% of AD patients are sensitised through IgE to common allergens, routine IgE monitoring during diagnosis is not necessary.
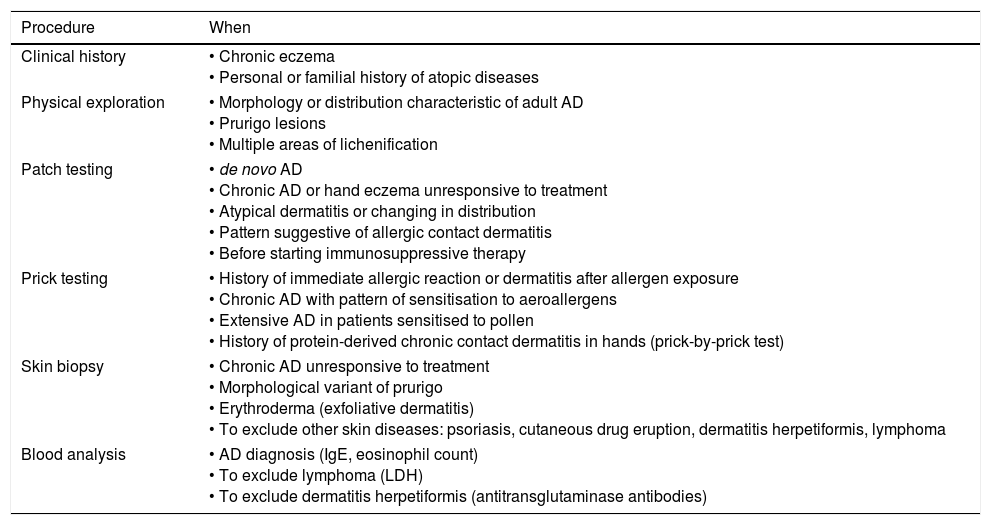
Adult AD diagnosis must search for possible comorbidities such as asthma, rhinitis, conjunctivitis and food allergies.16 However, AD in adults can present atypical morphology and localisation of skin lesions.17 Some forms of presentation that are observed in adults include, for example, head-and-neck dermatitis, chronic hand eczema, and multiple areas or lichenification or prurigo lesions.18 In Table 2 we show the procedures and tools currently available in the diagnosis of adult AD.18 A Chinese group of dermatologists have recently advanced a minimal set of diagnostic criteria for AD in adolescents and adults. These criteria were based on questionnaires and dermatological examinations of 2662 patients.19 They concluded that AD diagnosis must verify the presence of a symmetrical eczema (dermatitis) for more than six months plus one or more of the following:
- •
Personal and/or family history of atopic diseases
- •
Eosinophilia
- •
Elevated total serum IgE level and/or positive allergen-specific IgE.
Diagnostic procedures for adult AD patients.
| Procedure | When |
|---|---|
| Clinical history | • Chronic eczema • Personal or familial history of atopic diseases |
| Physical exploration | • Morphology or distribution characteristic of adult AD • Prurigo lesions • Multiple areas of lichenification |
| Patch testing | • de novo AD • Chronic AD or hand eczema unresponsive to treatment • Atypical dermatitis or changing in distribution • Pattern suggestive of allergic contact dermatitis • Before starting immunosuppressive therapy |
| Prick testing | • History of immediate allergic reaction or dermatitis after allergen exposure • Chronic AD with pattern of sensitisation to aeroallergens • Extensive AD in patients sensitised to pollen • History of protein-derived chronic contact dermatitis in hands (prick-by-prick test) |
| Skin biopsy | • Chronic AD unresponsive to treatment • Morphological variant of prurigo • Erythroderma (exfoliative dermatitis) • To exclude other skin diseases: psoriasis, cutaneous drug eruption, dermatitis herpetiformis, lymphoma |
| Blood analysis | • AD diagnosis (IgE, eosinophil count) • To exclude lymphoma (LDH) • To exclude dermatitis herpetiformis (antitransglutaminase antibodies) |
There are many indices developed to assess severity of AD described in the literature, mostly designed specifically to provide quantitative measurements in clinical trials. Routine clinical practice does not use severity scales to assess patients; however, collecting data regarding pruritus intensity, sleep disturbance or impact on daily activity, is encouraged.
The Scoring Atopic Dermatitis (SCORAD) cumulative index combines objective (extent and intensity of lesions) and subjective (daytime pruritus and sleep loss) criteria and it is widely used to quantify severity of the disease towards determining comparative efficacy of treatments and progression of the disease in clinical trials.20 Thus, SCORAD scores of <25 reflect a mild, transient, form of the disease; a score of 25–50, moderate or recurrent AD; and >50 a severe, persistent form of AD. The Eczema Area and Severity Index (EASI) is another validated and widely-used scale.21 Unlike SCORAD, EASI only takes into account the physician's objective criteria. POEM (Patient-Oriented Eczema Measure) quantifies symptoms over the period of a week and is designed to be completed by the child or parent; it has also been validated recently.22
Aetiology, physiopathology and epidemiologyAD can be associated with multiple comorbiditiesAD is often the first manifestation of a dysfunction of the immune system known as the ‘atopic march’ which is a well-described sequential appearance of AD, rhinitis and asthma in affected patients. AD in early childhood is strongly associated with asthma later in life.23 A large study of Swedish children found that almost half of the children with AD will go on to develop AD, asthma, or rhinitis in preadolescence. Surprisingly, children with AD in their first year of life, but not their second year of life, have a significantly lower risk of AD in preadolescence than do other children with infantile AD, although with an equally increased risk for asthma and rhinitis.24 AD in adults is strongly associated with allergic comorbidities: a recent study of 380 adult AD patients showed that 40.3% had asthma, 51.3% had allergic rhinitis, 24.2% had allergic conjunctivitis, and 60.5% had other allergic conditions.25
Patients with atopy also have a tendency to develop food allergies: one-third of children with AD have IgE-mediated clinical food allergy.26 AD and skin dysfunction is a major risk factor of food allergy even in exclusively breast-fed children.27 The causal relationship between food allergy and AD is still unclear mechanistically but AD precedes the development of food sensitisation and allergy, suggesting that one leads to the other.28 A trans-epidermal water loss test (TEWL) performed at birth (revealing skin barrier dysfunction) can predict food allergy at year two even in children without AD, supporting the concept of transcutaneous allergen sensitisation. This test (TEWL) could be used to stratify children before the development of AD or food allergies, and to take potential preventive measures.29 Food allergy is usually much less frequent in adult AD patients.
In the last decade, an increasing number of studies have associated AD to a surprisingly wide array of non-allergic comorbidities. For some of them, however, it is unclear if they are true comorbidities with an underlying causality, or secondary effects of AD.
AD has been associated to psychological and stress-related syndromes. For example, AD in early childhood can elevate the risk of developing attention deficit hyperactivity disorder (ADHD) and autistic spectrum disorder (ASD) later in life. Interestingly, the likelihood of ADHD or ASD correlates with the degree of intensity of the atopic disorder in childhood.30 A recent study found a similar strong association between AD and ADHD in children and adults in the US. Children with AD and severe sleep problems had a higher risk of ADHD, and also those with a history of anaemia, headaches and obesity. In adults with AD the presence of asthma, insomnia and headaches increased the odds ratio of ADHD, and low BMI was protective.31 Children with AD also had a significant risk for other mental disorders such as depression, anxiety, conduct disorder, and autism.32 A recent study of Korean adults has shown a positive correlation between perceived psychological stress and AD.33 In another study, using mouse models, it was shown that exogenous stress could reduce rather than increase inflammatory response during AD, probably due to an increased production of endogenous glucocorticoids.34 Chronic stress can suppress/dysregulate immune function but acute stress for short time-periods could have immune-enhancing effects.35
The evidence supporting the recognition of the nexus between AD and inflammatory and cardiovascular disease is more enigmatic. A study of German AD patients revealed increased risk of rheumatoid arthritis and chronic inflammatory bowel disease among them.36 It is unknown if these comorbidities result from the chronic inflammatory state or from a common immunological dysfunction.37 Epidemiological data support a connection between adult AD and increased rate of heart attacks and stroke, perhaps as a result of AD patients having a higher propensity to smoke, consume alcohol, and lead a sedentary lifestyle.38
In children, moderate and severe AD could be associated to obesity and increased high blood pressure. Although the mechanism is unknown, several studies have associated AD with alterations in adipose tissue and adipokines.39
AD risk is elevated also in patients with early-onset vitiligo or alopecia areata, especially alopecia totalis or alopecia universalis, suggesting a possible common underlying mechanism between AD and autoimmune diseases.40
Childhood AD often predicts AD in adulthoodFor most childhood patients AD resolves itself in adolescence. Recent estimates show that 80% of childhood AD does not persist by eight years and less than 5% persists by 20 years after diagnosis.41 However, some patients retain their condition after age 20. In general, the rate of persistence into adulthood correlates with the later the onset, the longer the persistence, and the stronger severity in childhood.41 In the USA, 10–30% of childhood AD patients go on to develop the disease in adulthood,3 and of these, 20% have moderate-to-severe symptomatology. In a Danish cohort study including approximately 1300 individuals aged 28–30 years who had been followed-up for 15 years, persistent AD was found in 50% of those diagnosed in school age, and was significantly associated with early onset, childhood allergic rhinitis and hand eczema.42
Asthma, rhinitis, conjunctivitis, food allergies and psychological comorbidities are common among adult AD patients.16 Indeed, 10% of adults with food allergy have concomitant AD, but the incidence of new-onset food allergy in adult AD patients is currently unknown. No clear risk factors have been observed for the development of adult AD but cumulative cigarette smoking or exposure to environmental tobacco in childhood could contribute to the development of adult-onset AD,43 and active and passive smoking has been found to be clearly associated to increased AD prevalence.44 According to an epidemiological study in India, the prevalence of adult-onset AD patients with mite sensitivity (positive skin prick tests) was high.45 Several studies in Asia revealed that 9–24% of AD patients had onset of the disease after 18 years of age.46 Interestingly, filaggrin mutations (see below) are not associated with late onset or adult AD.47
The increasing worldwide prevalence of AD has been associated to diverse risk factorsOver the last decade a large body of epidemiological data has been accumulated thanks to the International Study of Asthma and Allergies in Childhood (ISAAC), a global study that included almost two million children from 100 countries.48 This study has provided methodologically validated and uniform data from countries with very diverse lifestyles and healthcare standards, allowing for direct comparison of childhood AD prevalence in the world. The study showed that prevalence in children 6–7 could range from 0.9% in India to over 20% in some Latin American countries. Countries as diverse as Ecuador or Sweden had similar AD prevalence over 22%. The global prevalence was 7.9% among 6–7-year-olds, and in this age range Spain had a prevalence of 6.2%.
Interestingly, the ISAAC study showed that while in some more developed countries AD in children seems to have reached a plateau, in less-developed countries of Latin America and South East Asia, AD continues to increase in prevalence.48 Prevalence data in children show a slight female to male preponderance (1.3–1).49
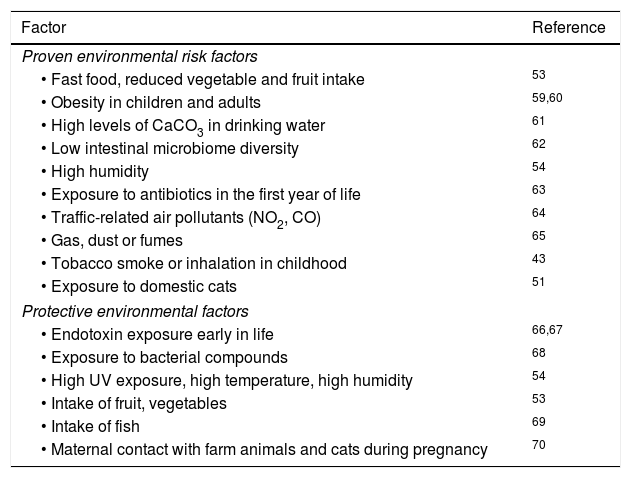
Attempts to correlate childhood geographic prevalence of AD show an important effect of the environment but have not yielded a specific causative factor.5 For example, exposure to farm animals during pregnancy and early childhood is associated with increased AD in children living in less-developed countries, but not in highly developed countries.50 Exposure to cats seem to increase AD risk worldwide,51 but exposure to dogs could be protective.52 Food intake, such as fruits, nuts and fish, clearly correlated with reduced AD.53 A study of US children showed that combined high UV exposure and high temperature is correlated with a lower incidence of AD.54 Studies so far do not offer any conclusive results with respect to AD incidence related to omega-3 fatty acids,55 childhood vaccinations, prolonged exclusive breastfeeding56 or viral/bacterial pathogens (Table 3).
Environmental risk and protective factors in AD.
| Factor | Reference |
|---|---|
| Proven environmental risk factors | |
| • Fast food, reduced vegetable and fruit intake | 53 |
| • Obesity in children and adults | 59,60 |
| • High levels of CaCO3 in drinking water | 61 |
| • Low intestinal microbiome diversity | 62 |
| • High humidity | 54 |
| • Exposure to antibiotics in the first year of life | 63 |
| • Traffic-related air pollutants (NO2, CO) | 64 |
| • Gas, dust or fumes | 65 |
| • Tobacco smoke or inhalation in childhood | 43 |
| • Exposure to domestic cats | 51 |
| Protective environmental factors | |
| • Endotoxin exposure early in life | 66,67 |
| • Exposure to bacterial compounds | 68 |
| • High UV exposure, high temperature, high humidity | 54 |
| • Intake of fruit, vegetables | 53 |
| • Intake of fish | 69 |
| • Maternal contact with farm animals and cats during pregnancy | 70 |
Adult AD has been much less studied epidemiologically. Although the estimated prevalence in adults is 1–3%,3 it is likely that the prevalence of severe AD is substantially lower. However, it is possible that mild or moderate adult AD is underdiagnosed because signs and symptoms tend to be less severe than in children, or because dermatologists are prone to diagnose allergic contact dermatitis or air-borne contact dermatitis rather than AD in some countries.46
In the elderly (>60 years) the prevalence is lower than in the younger population, with reports of 1.86% in Poland57 and 2.6% in Japan.58 AD is more common in elderly males than in females, with a proportion of 2:1.
AD can strongly affect all aspects of the life of patients and their familiesAD can have a huge impact on the quality of life (QoL) at any stage of life. The effects of the illness can secondarily result in profound emotional, psychological, economical, and social burdens on the patients and their families. Some examples of recent studies of the impact of AD in life are shown below:
- •
Family life and relationships can be severely disrupted in childhood AD. Quality of family life is related to the severity of AD in children.71 A recent study in Croatian children showed that family QoL is significantly correlated with the SCORAD score, patient-oriented SCORAD, itching, sleeplessness, and the Perceived Stress Score (PSS).72 There is limited evidence from included paediatric studies that educational intervention (multi-professional eczema interventions and nurse-led clinics) may lead to severity improvement and quality of life in children.73
- •
Sleep quality. Severity of the disease is highly correlated with sleep quality and QoL in children and adult AD. Evaluation of sleep quality should be necessary in the management of AD patients. For example, in Canada more than 50% of AD patients report sleep disturbances owing to their condition.74
- •
Psychological distress. A study performed on South Korean young adult males with AD has shown that there is a high association with depression, anxiety, and somatoform disorders.75 A large study of Europeans found that clinical depression, anxiety disorder, and suicidal ideation are very common in AD patients.76 A recent study showed that clinically relevant anxiety or depression was reported by 21.8% adult AD patients.25
- •
Bullying and low self-esteem in school children. According to a survey by the National Eczema Association carried out in 2016, 20% of the respondents (parents and caregivers) indicated that their children experienced bullying at school (National Eczema Association, 2016).
- •
Job choice and occupation. The International Study of Life with Atopic Eczema (ISOLATE) showed that up to 38% of adult AD patients report being affected by the illness in career choices, increased absenteeism rate, and early retirement. 10% reported discrimination in the workplace.
- •
Sexual life. The ISOLATE analysis showed that the AD adversely affected sexual relationships in more than half of the patients.77
- •
Social isolation. Often adult AD patients are isolated and avoid social or family activities because of their condition.77
AD is the most common skin disease in children globally. Since it has a deep impact on patient quality of life, the disease creates a substantial economic burden on the families affected. However, few studies have quantified in detail the economic effects of AD in healthcare systems. An estimate of overall yearly costs of AD in Canada in 2006 was of 1.4 billion (Canadian dollars).74 A more recent estimate indicated yearly costs of $5.297 billion in the US.78 A review study of the South East Asia region concluded that direct costs of AD per patient per year ranged from $199 in Thailand to $4842 in Australia.79 The components of direct costs for AD are medical visits and creams, dressing, ointments and medications. Indirect costs resulting from the disruptive effects of AD in family life (lost work from parents, transportation, nursing) are much more difficult to estimate. No recent detailed studies have been performed of the costs and burden of AD in Europe.
AD has a strong genetic componentAD is a complex and multifactorial disease in which genetic, immunological, environmental and skin barrier factors are closely entangled. Studies with twins have shown a heritability of AD of approximately 75%, revealing that genetic factors are key to understanding the aetiology of AD and other atopic diseases such as asthma.80 AD develops in 20–30% of children when one of the parents present an atopic disease, and in 40–50% when both parents are atopic.81
Two loss-of-function mutations in the filaggrin gene (FLG), R501X and 2282del4, have been identified as the strongest genetic risk factor for AD.82 These mutations are highly prevalent and 9% of the European population is estimated to be heterozygous, resulting in 50% less filaggrin in the skin.83 However, a recent study has shown that mutations in the FLG gene are associated only with early-onset AD, and not with late-onset or adult AD.47
Filaggrin expression can be modulated by a large number of factors, explaining why the loss-of-function mutations are only present in a fraction of AD patients.84 Most cases of moderate-to-severe AD present lower filaggrin gene expression85 and filaggrin monomer copy number variation also correlates with AD risk.86 Other factors affecting filaggrin expression are DNA methylation, environmental (dryness, skin irritants, sunburn), cytokines (IL-4, IL-13, IL-17A, IL-22, IL-25, IL-31, TNF-α), microorganisms (bacteria and parasites) and some topical therapies. It has been suggested that therapies oriented towards increasing filaggrin expression could be useful in the treatment of AD.86
Recent preliminary genetic evidence points to the innate immune system as playing a role in AD pathogenesis, as SNPs located in two inflammasome genes, NLRP3 and CARD8, showed a significant positive association to AD.87 No SNPs in NLRP1 were found associated to the disease in this study. Recently, impaired NLRP3 function was also implicated in the development of chronic skin inflammation in AD after Staphylococcus aureus colonisation and infection.88
A subset of AD patients can develop sensitivity to infection by Herpes simplex (eczema herpeticum, also known as Kaposi varicelliform eruption) or to molluscum contagiosum virus (eczema molluscatum). This susceptibility probably derives from an imbalance between antiviral immune responses and regulatory T cells.89 Indeed, some genetic variants in the interferon regulatory factor 2 (IRF2) were shown to be associated with AD.90
AD involves a severe disruption of the skin barrierThe skin is the first line of protection and defence of the body against environmental attack. The stratum corneum is the outermost layer of the skin, and it is composed of keratinocytes and a complex mixture of lipids (ceramides, cholesterol, and free fatty acids) and antibacterial peptides. The permeability barrier, determined by the lipids, and the antibacterial barrier, determined by peptides, are co-regulated and interdependent functions, and both are compromised in AD.91
A critical element of skin homeostasis is filaggrin, a protein generated from a large pro-filaggrin precursor during terminal differentiation of epidermal cells. Filaggrin serves a diverse number of functions in the skin, which explain its central importance in the development of AD. Filaggrin contributes to aggregation of keratin bundles intracellularly in keratinocytes, but it is also degraded extracellularly into a variety of metabolites. The natural degradation products of filaggrin, ultimately small polycarboxylic acids, are critical to keep adequate moisture in the skin and also determine morphology of corneocytes in the outer layers of the dermis. AD patients with reduced expression or loss-of-function filaggrin mutations in the FLG gene present abnormal corneocyte morphologies which partly explain the breakdown of the skin barrier. Filaggrin deficiency contributes to AD pathology not only by decreased hydration of the stratum corneum, leading to increased water loss, but also because the reduced polycarboxylic acids required to keep low pH are necessary to regulate serine protease activity.4 Serine proteases are required to digest corneodesmosomes and allow progressive degradation of the corneocytes in the stratum corneum. Claudin 1, a protein involved in tight junction formation, is also reduced in AD,92 which may be a factor enhancing herpes simplex virus 1 infection in AD patients.93
The skin microbiome could be a major player in AD pathogenesisIn the last ten years the human microbiome has come under intense focus as a critical aetiological component of a diverse number of diseases. The interaction of microbes with the immune system in the skin is clearly fundamental in the development of AD. Increased levels of S. aureus in the skin have been long associated with AD, possibly as a consequence of increased pH. Colonisation by S. aureus has been observed in 90% of AD patients, while only 5–30% of non-atopic individuals are colonised.94S. aureus in turn releases virulence factors such as the alpha toxin which, together with Th2 cytokines, result in keratinocyte apoptosis in AD lesions. Staphylococcus epidermidis or other staphylococci could play an important role in controlling skin AD infections by S. aureus.95 Treatment with emollients can change the bacterial composition of the AD lesions significantly, with Streptococcus, Propionibacterium, and Corynebacterium species increasing following therapy.96 It has been suggested that therapy-dependent changes in skin bacterial diversity are the reasons for the improvement in the disease.96 Indeed, treatment with corticoids partially restores skin microbial diversity, usually very low in AD patients.95
The possible benefits of probiotics for the prevention or control of AD have been extensively studied and, although many analyses point to a positive effect of probiotics in AD, the evidence so far remains controversial. A number of studies have pointed to bacterial compounds as potentially having a protective effect from AD in children. For example, children that were fed a lysate of Escherichia coli and Enterococcus faecalis prevented the development of AD, especially in children with paternal atopy.68
AD reveals a highly complex interaction between the skin and the immune systemIn addition to an altered skin barrier function and the skin microbiome, the third major actor in AD is the immune system. AD lesions have a characteristic increased numbers of T cells, dendritic cells, macrophages, mast cells, and eosinophils, as well as increased levels of the different cytokines and chemokines that these cells generate. Thus, components of both the innate and the adaptive immune response are implicated. Despite the highly complex network of functional interactions, a coherent picture of the complementary roles of the innate and adaptive immune system in AD is slowly emerging. These novel insights have in turn led to the development of novel therapies based on immunological regulation.
AD patients have an altered innate immune system. They have been found to have reduced TLR2 and TLR9 function.97 These innate immunity defects of the epidermal barrier repair process in AD patients colonised by S. aureus could lead to alteration in the skin microbiome and inflammation.96 While in normal skin antimicrobial peptides (AMPs) production is upregulated by two cytokines, IL-17 and IL-22, secreted by Th17 and Th22 cells, this effect is abrogated in atopic skin by the presence of Th2 cytokines.98
Inflammosomes are activated in keratinocytes after allergen exposure and also by S. aureus. NLRP3 expression is reduced in AD skin lesions, possibly in part by the action of IL-4, IL-5 and IL-13. Impairment of NLRP leads to chronic skin inflammation in the presence of S. aureus infection.88
The role of IgE in AD is still controversial as it is unclear if it plays a role in the pathogenesis or is a consequence of allergy.4 The acute phase of AD has been associated with increased expression of the major Th22 and Th2 cytokines, as well as cytokine-regulated genes involved in terminal cell differentiation.99 Keratinocyte differentiation is affected by altered expression of a large number of genes in AD lesions. For example, IL-4 and IL-13, detected in AD lesions, result in decreased filaggrin expression in keratinocytes. AD acute lesional skin is characterised by the overexpression of IL-4, IL-13 and Th17 cytokines, which lead to suppression of the expression of keratinocyte differentiation genes such as lorichrin and involucrin, and inhibition of the production of antimicrobial peptides.100,101 IL-4 and IL-13 promote expression of protease kallikrein 7 (KLK7) in keratinocytes. Enhanced protease activity is known to induce epidermal barrier dysfunction.102 As shown below, the neutralisation of IL-4 and IL-13 has proven to be a successful therapeutic strategy in AD.
IL-25 can inhibit filaggrin synthesis in keratinocytes. IL-25 could be the link between inflammation (Th2 response) and the skin integrity alterations typical of AD.103 In addition to filaggrin, cytokines elevate levels of thymic stroma lymphopoietin (TSLP) in keratinocytes, leading to skin dysfunction.102,104
Treatment of ADThere is a wide number of therapeutic options for mild and moderate ADThere is no standardised therapy for all cases of AD and treatment of moderate and severe cases, especially in their acute phase, can be challenging. Therapy must be personalised and options must take into account factors such as the age of the patient, severity of the disease, the appearance of flares, the existence of comorbidities, and psychological issues, among others. Current treatment aims to control three key aspects of the disease: skin barrier disruption, immune response, and microbial infection. Standard therapeutic options are the same for childhood or adult AD, especially for mild-moderate forms of the disease.
A baseline treatment for AD is moisturising skin cream to reduce water loss and help hydration of the stratum corneum in the affected areas. Optimal skin hygiene is essential in children and it is advisable to avoid any environmental factors that can trigger inflammation.1 Emollients (glycol and glyceryl stearate, soy sterols) provide water and lipids, which reduce inflammation. Occlusive agents (petrolatum, dimethicone, mineral oil) reduce water evaporation and recent evidence shows that they can also upregulate antimicrobial peptide production and induce expression of filaggrin and loricrin.105 Humectants (glycerol, urea) also help to retain water. The use of the different presentations of emollients (ointment, cream, lotion, or oils) depends on the type of skin lesion but issues such as time of application, optimal frequency, and quantity are not standardised and their efficacy difficult to estimate.106 Currently prescription emollient devices (PEDs) with complex mixtures that aim to mimic skin components are being developed to target specific lesions.107
For mild forms of the disease (SCORAD<25) the treatment options include topical glucocorticosteroids and topical calcineurin inhibitors (tacrolimus ointment and pimecrolimus cream).108 Topical glucocorticosteroids can be associated with significant adverse effects when used chronically, and can sometimes, if exceptionally, generate contact allergy.6
Moderate or recurrent forms of AD (SCORAD 25–50) require stronger topical glucocorticoids and calcineurin inhibitors, or phototherapy.107 Best results could be achieved by a combination of proactive therapy (long-term disease control by daily application of emollients to unaffected skin, combined with intermittent and minimal use of anti-inflammatory drugs) and reactive therapy on an ‘as needed’ basis (topical glucocorticoids and calcineurin inhibitors on affected skin).109 Clinical trials of proactive therapy with topical tacrolimus, fluticasone propionate and methylprednisolone aceponate have shown efficacy in preventing flares in the long term.110 Although proactive anti-inflammatory therapy is generally well tolerated, future clinical investigation will be required to determine its long-term safety.110
Phototherapy is a good treatment option and provides long-term control of the disease, with preference given to UVA1 and NB-UVB. Usually, phototherapy is advisable only in addition to topical anti-inflammatory and antimicrobial therapy.6 Phototherapy as a second-line treatment after maximising topical treatments and oral cyclosporine is also efficacious in children.111 Issues related to the child's fear of the cabin, loss of school time, and adverse effects must be considered.
Systemic antihistamines can prove effective to sedate and allow enhanced sleep, resulting in less nocturnal scratch, although they can affect sleep quality.1,112
Skin infection with S. aureus and a concomitant reduction in bacterial diversity is very common (90%) in moderate and severe cases of AD,96 and antimicrobial therapy is required. However, topical (or systemic) antibiotics should not be used for long periods, and only as necessary to treat infected acute flares.1 Due to the increasing rate of bacterial resistance to antibiotics, some clinicians have recommended the use of bleach baths to control infection,113 although their efficacy has proven controversial.114 Wet wraps are often used in the context of major flares to reduce severity of the disease. They can help increase the concentration and penetration of the topical agent used, and also protect against scratching.107
When the use of topical anti-inflammatory or UV therapies has been exhausted, moderate and severe AD can be treated with systemic glucocorticoids (methylprednisolone). However, the secondary effects of systemic glucocorticoids strongly limit their use to a few weeks. Systemic immunosuppressive therapies have been the mainstay of the management of moderate and severe AD cases, and they include treatment with cyclosporine A, azathioprine, mycophenolate mofetil, enteric-coated mycophenolate sodium, and methotrexate.115 Cyclosporin A is an effective treatment for children and adult AD, although attention should be paid to potential toxicities and interactions with other drugs.116 Cyclosporin clinical improvements were associated with significant gene expression changes in lesional and also non-lesional skin, as well as reductions in levels of certain cytokines.117 Intermittent short-course cyclosporine therapy in children can limit side effects and allows prolonging treatment.118 The off-label use of azathioprine is also recommended and its profile of side effects, which can be genetically determined, is well known.1,116 A recent survey of American and Canadian physicians showed a great variation in the use of immunomodulators for severe AD in children. Cyclosporine and methotrexate were the most common first-line drugs used.119
Alternative therapies to treat AD have been explored extensively but thus far their usefulness is controversial. Such therapeutic interventions include specific allergen immunotherapy,120 the use of Vitamin D,121 or Chinese herbal medicines.122 Similarly, there is no clear evidence that any dietary supplements such as probiotics or omega-3 oils are beneficial in the treatment of AD.123 However, recent data suggest that probiotics could help in the long-term prevention of AD.124
Novel small molecules have been introduced for the treatment of ADLeukocytes in AD patients have an increased phosphodiesterase-4 (PDE-4) activity, which is associated with the production of pro-inflammatory mediators. Apremilast is an oral drug developed for psoriasis but which has proven some effectiveness to control AD in a clinical trial with a small patient group.125 Recently several PDE-4 inhibitors of topical administration have emerged to treat AD specifically, such as crisaborole,126 E6005,127 and OPA-15406128 ointments.
Histamine-4 receptor is involved in the regulation of the production of Th2 chemokines in Langerhans cells in the skin and contributes to chronic inflammation in AD patients. Histamine-4 receptor antagonist JNJ39758979 has shown promising results in a preliminary clinical study of Japanese patients.129 ZPL-389 is another H4 antagonist is in Phase 2a clinical trials for moderate-severe AD, with some promising results.130
The Janus kinase (JAK) signal transducer is utilised by numerous cytokines (e.g., IL-4) acting on keratinocytes. Tofacitinib, a small-molecule JAK inhibitor, has been shown to be effective as an ointment in Phase IIa clinical trials for the topical treatment of AD.131
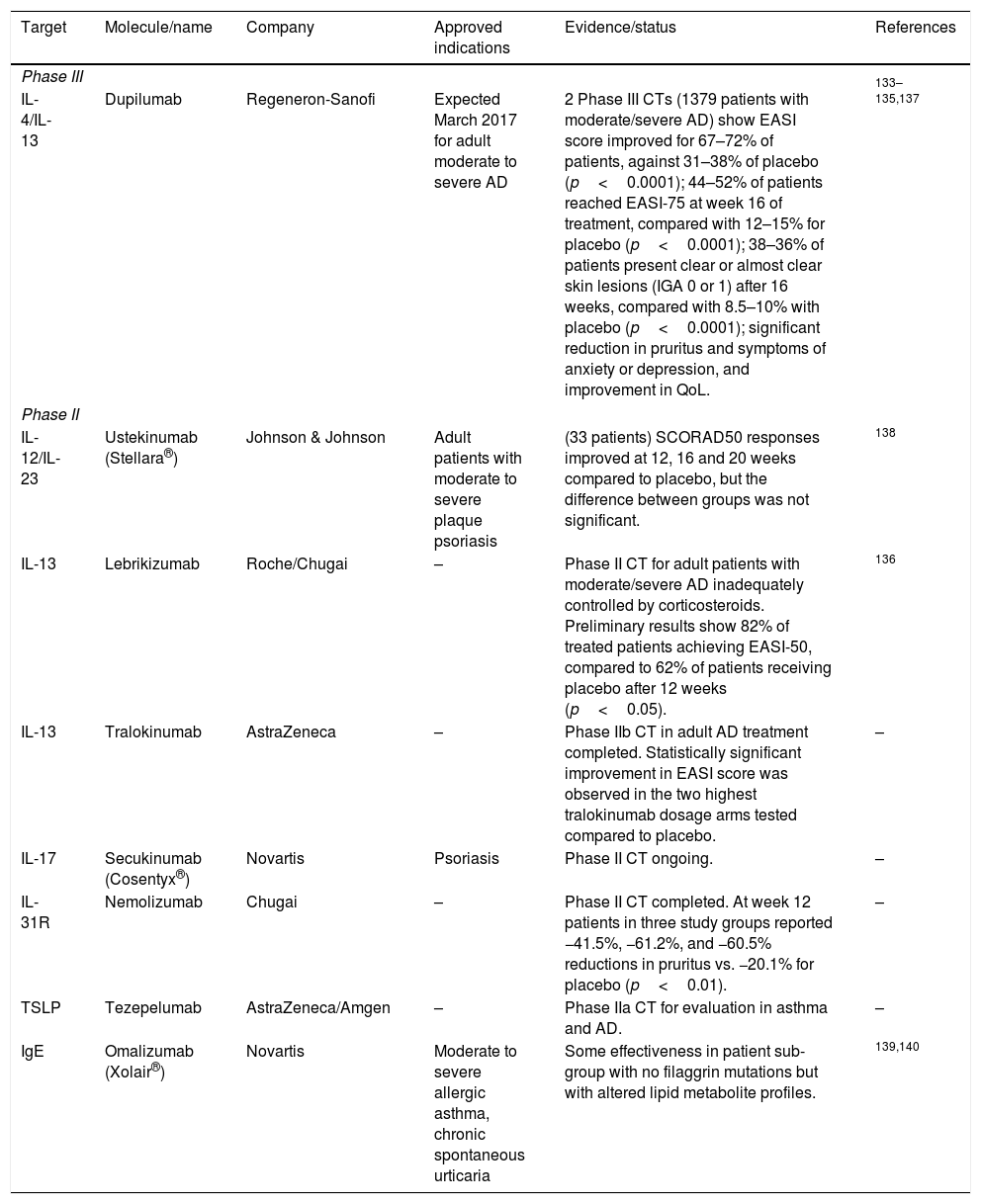
New biologicals open new horizons for the treatment of moderate and severe ADThe improved understanding of the involvement of the immune system in chronic inflammatory diseases such as AD has led to the development of novel immunoregulatory biologicals. Some of these had been tested in other inflammatory, allergic or autoimmune diseases and only recently have been clinically tested for AD, often with limited or inconsistent effectiveness.7,8 A summary of targets and novel biologicals and the evidence supporting their effectiveness is presented in Table 4.
Novel biologicals for AD therapy.
| Target | Molecule/name | Company | Approved indications | Evidence/status | References |
|---|---|---|---|---|---|
| Phase III | |||||
| IL-4/IL-13 | Dupilumab | Regeneron-Sanofi | Expected March 2017 for adult moderate to severe AD | 2 Phase III CTs (1379 patients with moderate/severe AD) show EASI score improved for 67–72% of patients, against 31–38% of placebo (p<0.0001); 44–52% of patients reached EASI-75 at week 16 of treatment, compared with 12–15% for placebo (p<0.0001); 38–36% of patients present clear or almost clear skin lesions (IGA 0 or 1) after 16 weeks, compared with 8.5–10% with placebo (p<0.0001); significant reduction in pruritus and symptoms of anxiety or depression, and improvement in QoL. | 133–135,137 |
| Phase II | |||||
| IL-12/IL-23 | Ustekinumab (Stellara®) | Johnson & Johnson | Adult patients with moderate to severe plaque psoriasis | (33 patients) SCORAD50 responses improved at 12, 16 and 20 weeks compared to placebo, but the difference between groups was not significant. | 138 |
| IL-13 | Lebrikizumab | Roche/Chugai | – | Phase II CT for adult patients with moderate/severe AD inadequately controlled by corticosteroids. Preliminary results show 82% of treated patients achieving EASI-50, compared to 62% of patients receiving placebo after 12 weeks (p<0.05). | 136 |
| IL-13 | Tralokinumab | AstraZeneca | – | Phase IIb CT in adult AD treatment completed. Statistically significant improvement in EASI score was observed in the two highest tralokinumab dosage arms tested compared to placebo. | – |
| IL-17 | Secukinumab (Cosentyx®) | Novartis | Psoriasis | Phase II CT ongoing. | – |
| IL-31R | Nemolizumab | Chugai | – | Phase II CT completed. At week 12 patients in three study groups reported −41.5%, −61.2%, and −60.5% reductions in pruritus vs. −20.1% for placebo (p<0.01). | – |
| TSLP | Tezepelumab | AstraZeneca/Amgen | – | Phase IIa CT for evaluation in asthma and AD. | – |
| IgE | Omalizumab (Xolair®) | Novartis | Moderate to severe allergic asthma, chronic spontaneous urticaria | Some effectiveness in patient sub-group with no filaggrin mutations but with altered lipid metabolite profiles. | 139,140 |
Abbreviations: CT, clinical trial; EASI, Eczema Area and Severity Index; IgE, Immunoglobulin E; IL, interleukin; QoL, quality of life; TSLP, thymic stromal lymphopoietin.
The targets of these new biologicals have been mostly the cytokines involved in Th2 allergic inflammation. Of these, the most successful have been those targeting IL4 and IL13.
Dupilumab is a fully human monoclonal antibody directed against the shared alpha subunit of the IL-4 receptors, blocking signalling for both IL-4 and IL-13.132 Transcriptomic analyses of pre-treatment and post-treatment skin biopsy specimens from patients with moderate-to-severe AD treated with dupimulab or placebo showed that dupilumab improved the AD molecular signature in a dose-dependent manner.133,134 Two recently published Phase III clinical trials, the SOLO 1 and SOLO 2 studies, showed improvements of the EASI score with respect to basal levels of 72% and 69%, respectively, for patients receiving 300mg of dupilumab weekly, and of 72% and 67% for patients receiving 300mg of dupilumab every two weeks, against improvements of 38% and 31% for patients with placebo (p<0.0001).135 In these two studies, a EASI-75 score was reached by 52% and 48% of the patients receiving 300mg of dupilumab weekly for 16 weeks, versus only 15% and 12% of the patients with placebo (p<0.0001).
Lebrikizumab, another antibody targeting IL-13, recently released results of a Phase II study in which the drug (125mg) was administered every four weeks on patients also treated with potent topical corticosteroids. The results showed that 82% of the patients reached EASI-50 scores at 12 weeks, compared with 62% on the same topical steroid regimen plus placebo (p<0.05).136
Biomarkers of severe AD could improve effectiveness of biological therapyAs a consequence of its complex and multifactorial aetiology, AD is highly heterogeneous. For example, up to 20% of AD patients with AD do not have IgE sensitisation.141 Also, loss-of-function mutations in the filaggrin gene only occur in a fraction of AD patients, although for various reasons, most or all patients present severe deficiency in this protein.84 This heterogeneity is reflected in the varied effectiveness of biologics targeting different inflammatory pathways. Thus, the anti-IgE biological omalizumab has shown good response on severe patients without mutations in filaggrin but not in patients with mutations.140
The heterogeneity of AD highlights the requirement for stratification of patients and also the urgent need for validated AD biomarkers. A recent systematic review and meta-analysis revealed that, despite a large number of studies and potential candidates, only a handful of biomarkers have potential for use in AD, namely serum thymus and activation-regulated chemokine (sTARC), serum cutaneous T-cell attracting chemokine (CTACK), sE-selectin, macrophage-derived chemokine (MDC), lactate dehydrogenase (LDH) and IL-18.15 The chemokine sTARC has been the subject of a number of studies as a biomarker of AD disease severity142 and has been shown in longitudinal and cross-sectional trials to be the most reliable biomarker to date.15 The level of sTARC can be used during early intervention in severe cases of children AD to evaluate activity and treatment efficacy.143 Finally, a recent study analysed the potential of thymic stromal lymphopoietin (TSLP), IL-31, IL-33 and soluble sST2 (a IL-1-receptor family-member that inhibits IL-33/ST2 signalling) as biomarkers of adult and childhood AD, and found that, although serum levels of TSLP, IL-31 and IL-33 are significantly elevated in AD patients, the level of these molecules cannot be used to assess disease severity.144
The clinical studies show a good safety profile of the new biologicals in the short termBecause of their specificity, biological agents are probably the least toxic and best tolerated approach to systemic therapy for AD. However, biologicals that target the immune system cytokines have not been tested in protracted treatments. Since cell signalling networks are exceedingly complex and interconnected, therapy with biologicals could potentially lead to adverse reactions in the long-term.
The current evidence shows that novel biologicals present a good safety profile. For dupilumab, a similar frequency of adverse events was observed for the drug and placebo groups,134,135 and side-effect profiles were not dose-limiting.137 Recent clinical trials with lebrikizumab showed a slight increase in Herpes infections and conjunctivitis.
Clinical trials for AD treatments are not fully standardised yet, and one common criticism is that the biologicals should be tested as monotherapy rather than on top of steroids, which might add confounding effects.
The new biologicals greatly improve quality of life in severe adult AD patientsCurrent thinking for AD therapy is shifting from the short-term treatment of flares to the long-term control of the disease.145 This is especially important for moderate and severe AD patients in whom, due to a lack of response to conventional immunosuppressive therapy and the impossibility of long-term treatment, the therapeutic options are very limited. For this reason, the emergence of new biologicals is very welcome, especially for those patients with a compromised quality of life because of moderate or severe AD. In this regard, patients treated with dupilumab, the only biological for which there are Phase III studies approved, had marked and rapid improvement in all the evaluated measures of AD activity. Dupilumab produced early and sustained patient-reported and clinically-relevant improvements in sleep, mental health, and health-related quality of life.146
AD in the elderly could be distinct from other age populationsAD is relatively uncommon in the elderly, but the population older than 65 is increasing worldwide, and therefore the number of older patients suffering from chronic conditions, including AD, is expected to rise. Prevalence of AD in the elderly could reach 1–3% in this population.57,58 AD in the elderly is of special concern due to the skin physiological changes of the ageing itself (xerodermia), comorbidities, and polypharmacy. Additionally, many clinical trials restrain the participants’ age to 65, thus limiting the information obtained on efficacy or adverse events in this patient group.
Diagnosis in the elderly may be difficult due to the side effects of concomitant treatments for hypertension, cardiovascular diseases, gastrointestinal disorders or diabetes mellitus, which could generate skin itchiness.58 Treatment options for the elderly are similar to adult AD but osteoporosis, hypertension and metabolic side-effects limit the use of systemic corticosteroids. Cyclosporin is also limited by hypertension or renal impairment, especially in patients older than 65.
AD treatment during pregnancy and lactation requires special precautionsAD shows similar symptomatology in pregnant women as compared to other patient groups.147 However, many patients typically report worsening of AD symptoms during pregnancy, especially in the second or third trimesters. A number of considerations must be taken into account when treating individual pregnant patients.147 Safe therapies for pregnant AD patients include emollients, topical corticosteroids, oral antihistamines, and UV light. Caution must be used when using oral corticosteroids, cyclosporine, azathioprine, topical calcineurin inhibitors. Methotrexate and mycophenolate mofetil should be avoided.147 Pregnancy labelling recommendations by the FDA have been revised recently and special precautions must be taken when treating pregnant patients. As a general rule, considering the potential risks and benefits, clinicians should avoid any systemic treatment in pregnant women, especially during the first trimester. Systemic corticosteroids are not recommended in the first trimester because of increased risk of cleft palate.
A recent study has shown that psychological stress (pregnancy, anxiety) during pregnancy increases risk of AD in the new born, possibly through the presence of abnormal steroid levels and oxidative stress.148
There is no evidence yet that dietary intervention (omega-3 fatty acids, cow milk, egg, or peanut allergens) by the mother during pregnancy or during breast-feeding will change the chance of development of AD by the child.55,149
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this study.
FundingEditorial assistance provided by Sanofi-Genzyme Spain.
AuthorshipAll authors equally made substantial contributions in the conception and design of the study, in writing the article and gave the final approval of the version submitted.
Conflict of interestES-B: Sanofi advisory board.
JOF: Sanofi advisory board.
IJ: Sanofi advisory board.
JCAH: Sanofi advisory board.
JFS: Sanofi advisory board and travel grants.
AMS: Sanofi advisory board.
LH: Sanofi advisory board.
AV: Sanofi advisory board.
JS: Sanofi advisory board.
Francisco López de Saro for editorial assistance.