Food allergy can have a major impact on quality of life of children and their parents. Questionnaires have been developed to measure the impact of this disorder. We aimed to validate the EuroPrevall questionnaire on Food Allergy-Quality of Life Questionnaire, Parent Form (FAQLQ-PF) and the Food Allergy Independent Measure (FAIM), translated into Spanish.
MethodsThe internal consistency of the FAQLQ-PF and the FAIM, translated into Spanish (Spain) and completed by the parents of 74 children with IgE-mediated food allergy, were evaluated with Cronbach's alpha. To test construct validity of the FAQLQ-PF, its correlation with the FAIM was also calculated. To assess their discriminant validity, we compared the values of both depending on the number of offending foods and for children with and without anaphylaxis.
ResultsThe values of Cronbach's alpha for the three domains in the FAQLQ-PF were over 0.9. The value of alpha for FAIM questions was below 0.6, which was attributed to the wording of one question. When this question was removed, alpha increased to over 0.70. There was a significant correlation between the FAQLQ-PF score and the FAIM. There were significantly poorer FAQLQ-PF scores in children with more food allergies and worse FAIM in those who had had anaphylaxis.
ConclusionsThe Spanish version of the FAQLQ-PF had a good internal consistency, good construct validity and validity to discriminate patients with more food allergies and anaphylaxis. It can be used as a tool to evaluate and monitor the quality of life in families with food allergic children.
Food allergy has been on the increase in the last decades, both in children and in adults.1 Severity is very variable, ranging from mild oral allergy syndrome to life-threatening reactions and, thus, mortality is not exceptional. This causes stress and limitations in life patterns on patients with food allergy and also in their families.
Tools have been developed to measure and monitor the impact of food allergy, and they are also useful to evaluate the effect of therapeutic measures such as techniques of desensitisation or other forms of immunotherapy.
EuroPrevall was a project to study different aspects of food allergy in Europe, funded under the Framework Programme FP6 of the European Union.2 This initiative developed several questionnaires on quality of life in food allergy (FA-QoL).3–6 The questionnaires have been translated and validated in several languages.7–12 The aim of our study was to validate the questionnaire on Food Allergy-Quality of Life Parent Form (FAQLQ-PF) and the Food Allergy Independent Measure (FAIM) translated into Spanish (Spain).
Material and methodsAs the FAQLQ-PF and the FAIM are intended for clinical work and communication between patients and health professionals, the English version was translated into Spanish by one of the authors, a paediatric allergist with extensive experience and daily clinical contact with children with food allergy and their parents. The initial version was reviewed by the rest of the authors, and the final version was agreed upon by all of them. The final version is available in the online supplement.
The questionnaire was completed by parents of 0–12-year-old children with food allergy. These had been diagnosed with active IgE-mediated food allergy as they had symptoms compatible with a food allergic reaction together with a positive skin prick test and/or serum specific IgE. The questionnaire was completed in our outpatients’ clinic when children attended for a challenge with one of the culprit foods, and prior to knowing the outcome of the challenge. There was no selection of patients based on age, food or severity, so the whole spectrum of food allergy could be present.
Parents completed the questionnaire following the instructions therein, with no influence of the authors. Parents were instructed to ask only if there was something they did not understand; just one question arose, in which the father of one of the patients asked what anaphylaxis was.
The FAQLQ-PF consists of 30 questions, divided into three domains: Emotional Impact (13 questions), Food Anxiety (8 questions), Social/Dietary Restrictions (9 questions), scored with a Likert scale from 0 to 6, with higher values associated to poorer quality of life. The classification of questions according to domain is shown in Tables E1 to E3 in the online supplement. Some of the questionnaires are intended for parents of children of all ages (Section A), some only for those 4–12 years of age (Section B), and others for those 8–12 years (Section C). The mean score of the questionnaire was calculated dividing the sum of values by the number of answered questions.
Section D part 2 included FAIM (Food Allergy Independent Measure), a tool that has been developed and proposed to evaluate construct validity of FA-QoL questionnaires and has shown good face validity, relevance and reliability.13 There is a set of four questions about parents’ feelings, and a set of the same questions about children's feelings according to parents’ opinions, that is, parents answer what they think that the children feel. Section D also included information about sex, age, number and type of eliciting foods, type and severity of reactions. The use of the questionnaire was allowed by the Ethics Committee of our centre and anonymity of answers was warranted.
To test the internal consistency of the FAQLQ-PF and FAIM, Cronbach's alphas were calculated. The corrected item-total correlations were also calculated, as well as Cronbach's alpha if an item was deleted. The correlations of questions within each domain were assessed. To evaluate construct validity of the FAQLQ-PF, the correlation of the mean score of all domains with FAIM values was determined. The non-parametric Kruskal–Wallis test was used to compare values according to the number of offending foods and the presence of anaphylaxis, both for the FAQLQ-PF and the FAIM, to assess their discriminant validity. The software programme SPSS 15.0 (Chicago, Ill, USA) was used.
ResultsThe sample comprised parents of 74 children (47 male, 27 female) with IgE-mediated allergy to food, with a mean±SD age of 6.46±3.2 years (range from eight months to 12 years and one month). Seventeen (23%) had been diagnosed with anaphylaxis. Twenty-five children (33.8%) were allergic to one food, and 49 (66.2%) had allergy to two, or up to eleven foods. The questionnaires were completed by 66 mothers, seven fathers, and in one case gender was not recorded.
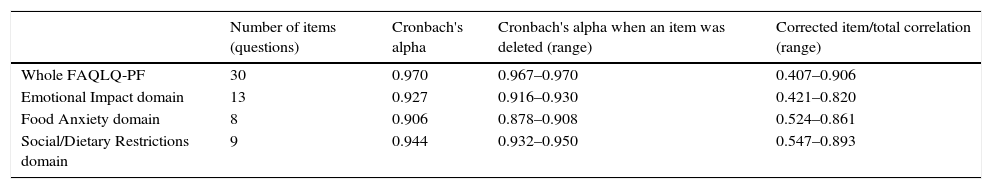
Value of Cronbach's alpha for the whole questionnaire (30 questions on three domains) was very high, 0.970. The values of alpha when each item was deleted had very little changes (0.967–0.970) and the corrected item/total correlation varied from 0.407 to 0.906. In general, the recommendation is to remove one item from the questionnaire if Cronbach's alpha has a marked increase and the corrected item/total correlation has a value <0.30.
The values of alpha for the subscales of the domains on Emotional Impact (0.927), Food Anxiety (0.906) and Social/Dietary Restrictions (0.944) were also very high, with no relevant changes when each question was deleted (Table 1). The values for each individual item are shown in the online supplement (Tables E1–E3).
Values of Cronbach's alpha for the whole questionnaire and for the subscales of each domain.
| Number of items (questions) | Cronbach's alpha | Cronbach's alpha when an item was deleted (range) | Corrected item/total correlation (range) | |
|---|---|---|---|---|
| Whole FAQLQ-PF | 30 | 0.970 | 0.967–0.970 | 0.407–0.906 |
| Emotional Impact domain | 13 | 0.927 | 0.916–0.930 | 0.421–0.820 |
| Food Anxiety domain | 8 | 0.906 | 0.878–0.908 | 0.524–0.861 |
| Social/Dietary Restrictions domain | 9 | 0.944 | 0.932–0.950 | 0.547–0.893 |
The correlations between questions within each domain are shown in Tables E4–E6. All pairs of questions in the domain of Social/Dietary Restrictions had significant correlations (r 0.366–0.956). A very high correlation (r>0.80) could mean that the information provided by the two questions in the pair is very similar (multicollinearity) and removal of one of them should be considered. These high correlations appeared in the following pairs of questions: “limitations in family holidays vs. visiting restaurants”, “food activities at school vs. social activities at others’ homes”, “food activities at school vs. limitations in family outing activities”, “exclusion in food activities vs. limitations in family outing activities”. There were also r values quite close to 0.80 in other pairs of questions.
All pairs of questions in the domain of food anxiety had significant values (r 0.405–0.779) except one, the pair “anxiety about food vs. worry for inadequate labelling of foods” (r=0.05).
Regarding the domain of emotional impact, as shown in Table E4, there were 16 out of 78 pairs of questions that did not have a significant correlation. The question about “wishing the allergy to disappear” had a significant correlation with just two of the other 12 questions in this domain, and the question on “physical suffering” with six questions.
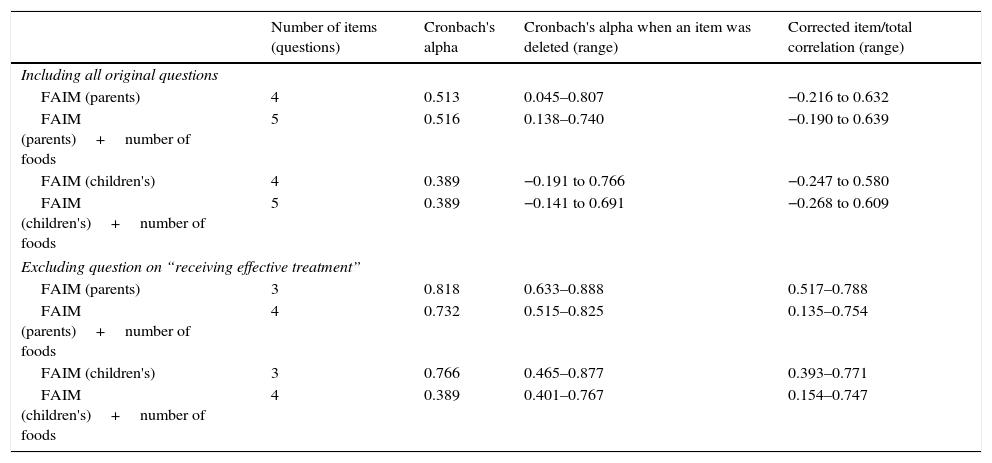
To estimate Cronbach's alpha for FAIM, the values on one of the questions (What chance do you think your child has of effectively treating him/herself, or receiving effective treatment from others – including Epipen administration – if he/she accidentally ingests a food to which he/she is allergic?) were reversed, as in the original questionnaire it had the opposite direction to the rest of the questions. That is, the higher values in the rest of the questions indicate worse expectations on reactions, but in that question a high value would indicate better expectations. Nevertheless, despite reversing the values, Cronbach's alpha was very low for parents’ FAIM and for children's FAIM, as shown in Table 2. We calculated Cronbach's alpha deleting that question, and then it reached values over 0.70. Including the question on number of foods causing allergy into the FAIM did not improve the test, and according to recommendations it should be rather removed. The specific values for each question are shown in Tables E7 and E8 in the online supplement. Additionally, this was supported by the lack of correlation of this question with the others (data not shown).
Values of Cronbach's alpha for FAIM questions (parents’ and children's).
| Number of items (questions) | Cronbach's alpha | Cronbach's alpha when an item was deleted (range) | Corrected item/total correlation (range) | |
|---|---|---|---|---|
| Including all original questions | ||||
| FAIM (parents) | 4 | 0.513 | 0.045–0.807 | −0.216 to 0.632 |
| FAIM (parents)+number of foods | 5 | 0.516 | 0.138–0.740 | −0.190 to 0.639 |
| FAIM (children's) | 4 | 0.389 | −0.191 to 0.766 | −0.247 to 0.580 |
| FAIM (children's)+number of foods | 5 | 0.389 | −0.141 to 0.691 | −0.268 to 0.609 |
| Excluding question on “receiving effective treatment” | ||||
| FAIM (parents) | 3 | 0.818 | 0.633–0.888 | 0.517–0.788 |
| FAIM (parents)+number of foods | 4 | 0.732 | 0.515–0.825 | 0.135–0.754 |
| FAIM (children's) | 3 | 0.766 | 0.465–0.877 | 0.393–0.771 |
| FAIM (children's)+number of foods | 4 | 0.389 | 0.401–0.767 | 0.154–0.747 |
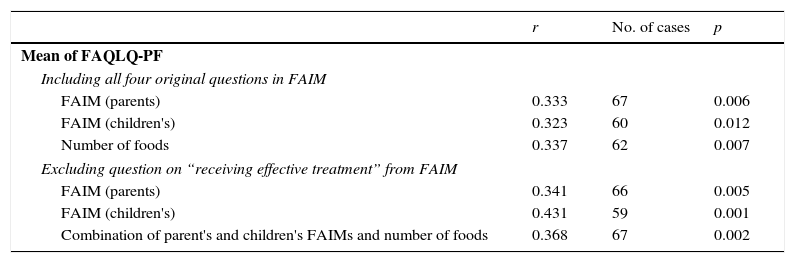
The correlation of the mean score of all valid questions in the three domains of the FAQLQ-PF with both parents’ and children's FAIMs was significant, when FAIMs included the three recommended questions but also when it included the question on receiving effective treatment. In fact, the values of r for both instances did not differ much, as shown in Table 3. The mean score was also correlated to the number of culprit foods and to the combination of both FAIMs together with the number of culprit foods.
Correlation of the mean value of valid questions in the three domains of the FAQLQ-PF with FAIM (the number of cases varies due to unanswered questions and to those not corresponding to age).
| r | No. of cases | p | |
|---|---|---|---|
| Mean of FAQLQ-PF | |||
| Including all four original questions in FAIM | |||
| FAIM (parents) | 0.333 | 67 | 0.006 |
| FAIM (children's) | 0.323 | 60 | 0.012 |
| Number of foods | 0.337 | 62 | 0.007 |
| Excluding question on “receiving effective treatment” from FAIM | |||
| FAIM (parents) | 0.341 | 66 | 0.005 |
| FAIM (children's) | 0.431 | 59 | 0.001 |
| Combination of parent's and children's FAIMs and number of foods | 0.368 | 67 | 0.002 |
When we compared the scores of the three domains and the FAIMs depending on the number of avoided foods we found differences for the domains score, but not for FAIMs, as seen in Table E9. There seemed to be differences in FAIMs between group 1 (avoid 1–2 foods) and the other three groups (avoid >2 foods); we gathered these three groups into one and compared with group 1, but the comparison remained non-significant.
Patients with anaphylaxis had significantly worse FAIMs and nearly significant worse mean score in the FAQLQ-PF than those without anaphylaxis; there were, however, no differences with regard to having been prescribed self-injectable epinephrine (Table E10).
DiscussionThe use of questionnaires on quality of life has been recommended for the evaluation and monitoring of patient-oriented outcomes in many areas of the field of health. Common questionnaires are useful for comparisons across countries, but the validation in different languages is needed.7–12 We have evaluated a Spanish version of the EuroPrevall FAQLQ-PF and found it to have good internal consistency, construct validity and discriminant validity.
To test the internal consistency of a questionnaire the value of Cronbach's alpha coefficient is recommended.14 Values >0.70 are acceptable, values >0.80 are considered good, and values >0.90 are excellent although they should raise concern about the possible redundancy of some questions.14 The value of alpha also tends to be higher with a larger number of questions, so testing Cronbach's alpha for each of the domains is recommended. We have found values also >0.90 for the individual domains. Some conditions (important change in alpha and corrected item/total correlation) are suggested to consider removal of items, but this happened with none of the questions.
On the other hand, the independent measure FAIM was also evaluated, and the value of Cronbach's alpha was <0.60. This measure has been described to be valid and relevant.13 We attributed our results to the wording of the question on receiving effective treatment in the event of a food reaction, quoted in “Material and methods” section. The other questions in FAIM have a negative direction, that is, high values are associated to worse impact and expectations in the family. In contrast, that question in the original form had a positive direction, that is, high values are associated to a beneficial impact on the family. We found a low value for Cronbach's alpha even though for the analysis we used the reversed answers. We attributed our results to confusion by this question; probably some parents may have followed the literal direction and others may have reinterpreted the question in the opposite direction (the same as in the rest of questions), making answers unreliable. This issue has been found previously, and the use of the negative form of the question has been recommended.13 Thus, in the presented translation in the online supplement, we have changed the wording making it negative, “…not treating … or not receiving effective treatment …” instead of “…treating … or receiving effective treatment …”.
When we removed that question, the value of alpha for the remaining items increased to over 0.70, except when we used the children's FAIM combined with the number of avoided foods. Moreover, the FAIM regarding children's feelings, answered by their parents, must be, evidently, greatly influenced by the age of children. Approximately 63% of the children were older than five years, and there were responses for children's FAIM in 80% of the questionnaires. We wonder how reliable the responses of parents interpreting their young children's feelings are, and whether the responses really reflect parents’ or children's feelings, as the correlations of the same questions for parents and children had high r values, between 0.660 and 0.770, meaning that coincidence appeared very often.
In spite of all these drawbacks with FAIM, there were significant correlations of the mean score of the three domains of the FAQLQ-PF with all the modalities of FAIM, regardless of including or excluding the question on receiving effective treatment, thus showing good construct validity.
When we compared results according to the number of avoided foods, the differences were significant for the mean FAQLQ-PF domains score but not for the FAIMs. A higher number of avoided foods can impact on everyday life, but not so much on the fears of reactions and their consequences, as measured by FAIMs. Probably the risk of finding a ubiquitous food and the severity of previous reactions are more important to affect FAIM.
We found poorer FAIM scores in patients who had had anaphylaxis, but not when we tested the fact of having been prescribed epinephrine, as would have been expected. This might be interpreted as a generous prescription of epinephrine as a precautionary measure although previous reactions had not been very severe. In other studies, discrepant results have been found.9
In summary, the Spanish translation of the FAQLQ-PF, similar to questionnaires translated into other languages7–12 showed good internal consistency, good construct validity when compared to FAIMs, and the ability to discriminate patients with more food allergies. However, the FAIM, although able to discriminate patients with anaphylaxis, merits re-evaluation with changes in the wording of the question on receiving effective treatment. Additionally, the FAIM on children's feelings would need re-evaluation and probably will have limitations depending on the age of the patients.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
FundingNone.
Conflict of interestNone.