Nowadays, the awareness of risks related to infectious diseases has decreased, whereas THE perception of risks related to vaccination is growing. Therefore, it may be difficult for health care providers to convince people of the importance of vaccination and adherence to the immunisation schedule.
Selected situations that might raise uncertainties about vaccine recommendations are discussed in order to help health care providers to identify real and perceived contraindications to vaccines, and cases to be referred to specialised pre-vaccination consultation due to an increased risk of adverse events to vaccines.
Nowadays, the incidence of several infectious diseases and, consequently, awareness of infection-related risks has decreased notably, whereas the perception of risks related to health interventions, especially vaccinations, is increasing. Now, more than ever, people want to have an active role in the protection of their own health. Therefore, health-care providers should be able to give people complete information on the importance of immunisation. This is, however, sometimes a difficult task.
The most common clinical conditions which may raise doubts about vaccine recommendations are discussed below. The aim of this review is to facilitate identification of those situations that are true contraindications to vaccination, and disorders requiring specialised pre-vaccination consultation due to an increased risk of adverse events to vaccines.1,2
Contraindications, precautions and warningsBefore administrating a vaccine, health personnel must verify if there are diseases that create risks of serious adverse events present in the vaccinee (“Contraindications” – see Table 1), or conditions which are more likely to be associated with adverse events or decreased vaccine efficacy: the vaccination may be offered with precautions in these cases, after assessment of risks versus benefits (see Table 2). Situations should be further identified in which the vaccine is safe and effective, but might interfere with an ongoing, or a recently stopped drug therapy (“Warnings for vaccination” – Table 3). Data on patient's medical history collected according to a standardised, specifically arranged format, are suitable in most cases for this scope. Evidence is lacking regarding the possibility of decreasing the risk of adverse events by clinical evaluation, or any diagnostic tests performed in apparently healthy subjects; therefore they are not recommended.
Contraindications to vaccination.
| For all vaccines |
| Anaphylactic reactions to vaccine components |
| Serious adverse events to a previous dose of vaccine |
| Anaphylactic reactions to the same vaccine or same vaccine components but low/moderate risks due to that specific infectious disease and its complications If the risk of infection for potentially lethal or disabling diseases is high, and specific antibody titres are not protective, the patient should be referred to an allergist to undergo skin tests with the vaccine and its components. If vaccine or vaccine component skin test results are positive, the vaccine might still be administered in graded doses according to a standardised protocol, in a hospital setting. |
| Patient who had complications requiring long-term or invasive treatment after previous vaccination and risks related to the disease to be prevented are low/moderate |
| Patient with complications without restitutio ad integrum, with low/moderate risks related to the disease to be prevented |
| For live attenuated vaccines: |
| Agammaglobulinaemic syndrome |
| Complete T-cell deficiency |
| HIV immunodeficiency with CD4+ lymphocyte count <15% of total lymphocyte count |
| Solid or haematological neoplasia during treatment |
| Transplant within six months |
| Current therapies |
| Chemo/radiotherapy up to six months from suspension |
| High-dose steroids for at least two weeks and up to one month from suspension |
| High-dose immunosuppressive drugs |
| Pregnancy |
| Contraindications concern live attenuated vaccines Varicella, Measles-mumps-rubella and Calmette-Guerin Bacillus and are related to the paucity of clinical trials. Accidental administration of Measles-mumps-rubella vaccine in pregnant women did not demonstrate an increase of teratogenic risk and it is not an indication for pregnancy interruption. |
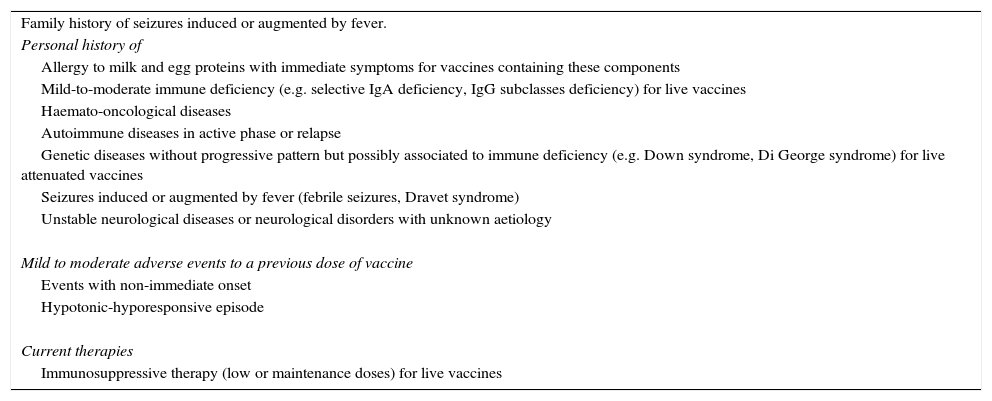
Precautions to vaccination.
| Family history of seizures induced or augmented by fever. |
| Personal history of |
| Allergy to milk and egg proteins with immediate symptoms for vaccines containing these components |
| Mild-to-moderate immune deficiency (e.g. selective IgA deficiency, IgG subclasses deficiency) for live vaccines |
| Haemato-oncological diseases |
| Autoimmune diseases in active phase or relapse |
| Genetic diseases without progressive pattern but possibly associated to immune deficiency (e.g. Down syndrome, Di George syndrome) for live attenuated vaccines |
| Seizures induced or augmented by fever (febrile seizures, Dravet syndrome) |
| Unstable neurological diseases or neurological disorders with unknown aetiology |
| Mild to moderate adverse events to a previous dose of vaccine |
| Events with non-immediate onset |
| Hypotonic-hyporesponsive episode |
| Current therapies |
| Immunosuppressive therapy (low or maintenance doses) for live vaccines |
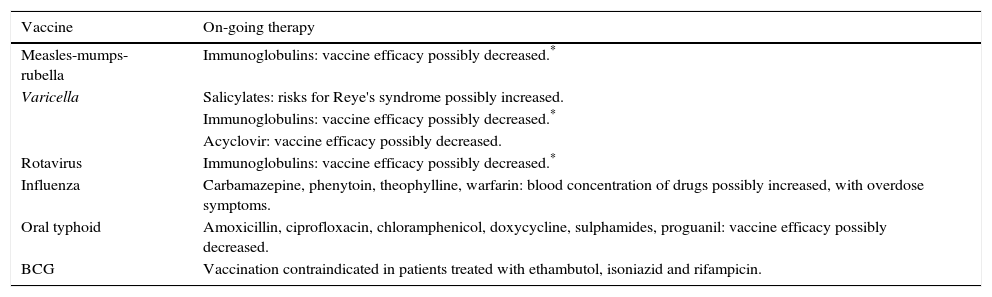
Possible interactions between vaccines and other therapies (warnings).
| Vaccine | On-going therapy |
|---|---|
| Measles-mumps-rubella | Immunoglobulins: vaccine efficacy possibly decreased.* |
| Varicella | Salicylates: risks for Reye's syndrome possibly increased. |
| Immunoglobulins: vaccine efficacy possibly decreased.* | |
| Acyclovir: vaccine efficacy possibly decreased. | |
| Rotavirus | Immunoglobulins: vaccine efficacy possibly decreased.* |
| Influenza | Carbamazepine, phenytoin, theophylline, warfarin: blood concentration of drugs possibly increased, with overdose symptoms. |
| Oral typhoid | Amoxicillin, ciprofloxacin, chloramphenicol, doxycycline, sulphamides, proguanil: vaccine efficacy possibly decreased. |
| BCG | Vaccination contraindicated in patients treated with ethambutol, isoniazid and rifampicin. |
If true contraindications to vaccination are identified (Table 1), vaccination should be avoided and the patient is to be referred to specialised consultation where available. If a risk/benefit assessment is indicated, health personnel should consider: the vaccinee's risk of contracting the infectious disease to be prevented; incidence and seriousness of complications associated with the infectious disease, taking into account all the patient's individual factors; levels of specific antibody titres to estimate protection; efficacy of the specific vaccine dose to be administered, and incidence and seriousness of adverse events associated with the vaccine. Consulting summaries of vaccine product characteristics and published guidelines, e.g. “Guide to Contraindications of Vaccinations”,3,4 is useful in decision-making: all contraindications, precautions and warnings are listed in these references. Updates of both general and specific guidelines about vaccinations should be consulted as well.
True contraindications to vaccinationsContraindications to vaccines (Table 1) are as follows:
- 1.
A personal history of previous anaphylactic reactions to vaccine components or substances used in the vaccine production.3,4 This is the only true contraindication applicable to all vaccines. An anaphylactic reaction is defined by a number of symptoms and signs involving at least two organs or systems among skin and cardiovascular, respiratory and gastrointestinal systems.5 By definition, an anaphylactic reaction is caused by mastocyte degranulation and it is very likely to occur in the presence of respiratory and/or cardiovascular symptoms. Although an anaphylactic reaction due to vaccines might occur up to 4h after administration, it generally occurs within 1h.6–8 Since anaphylactic reactions are life-threatening, vaccinations with the same product or vaccines sharing any possibly implicated substances must be avoided when disease-related risks are mild to moderate. If the risk of infection for potentially lethal or disabling diseases is high, and specific antibody titres are not protective, the patient should be referred to an allergist to perform skin tests with the vaccine and its components.7 If vaccine or vaccine component skin test results are positive, the vaccine may still be administered, if necessary, in graded doses. If the full vaccine dose is normally a volume of 0.5mL, the patient is first given 0.05mL of a 1:10 dilution and then given full-strength vaccine (at 15-minute intervals) at doses of 0.05, 0.1, 0.15, and finally 0.2mL, for a cumulative dose of 0.5mL. In a patient who is presumed to be allergic to the vaccine being administered, this procedure needs to be performed in a hospital setting under direct medical supervision, with emergency medications and equipment immediately available to promptly treat an anaphylactic reaction should it occur.7,9 We recommend an allergological consultancy before immunisation in any cases of possible IgE-mediated reactions to vaccines (e.g., immediate symptoms limited to the skin or mucosae, with or without cardio-respiratory involvement).
- 2.
Conditions of severe immunodeficiency: Severe primary or acquired immunodeficiency, such as agammaglobulinaemia, complete T-cell deficiency, HIV infection with CD4+ count less than 15% of total lymphocyte count, pharmacologically-induced immune depression (chemotherapy and radiotherapy up to 3–6 months upon suspension, high-dose steroids for 2 weeks and up to 1 month upon suspension, chronic therapy with immunosuppressive drugs), patients within 6 months of organ transplants.10,11 Contraindications refer to live attenuated vaccines, due to risks of clinically relevant infections by vaccine strain viruses in these patients.10 In less severe, or mild immunodeficiency disorders (e.g. selective IgA deficiency, partial Di George syndrome), live attenuated vaccines may be administered after an accurate risk/benefit assessment.
- 3.
Previous serious, adverse reactions to vaccination different from anaphylaxis. Vaccine adverse events are considered serious if they result in death, or are life-threatening, require hospitalisation or prolongation of existing hospitalisation, result in persistent or significant disability/incapacity, or required intervention to prevent permanent impairment or damage.12 Examples of serious adverse events are thrombocytopenia, Guillain-Barrè syndrome, or brachial neuritis. In these cases, further doses of the same vaccine should be avoided, unless benefits clearly overcome risks.3 The vaccination might be offered with personalised precautions, if risks for a potentially lethal or disabling disease are high and patients give their informed consent.
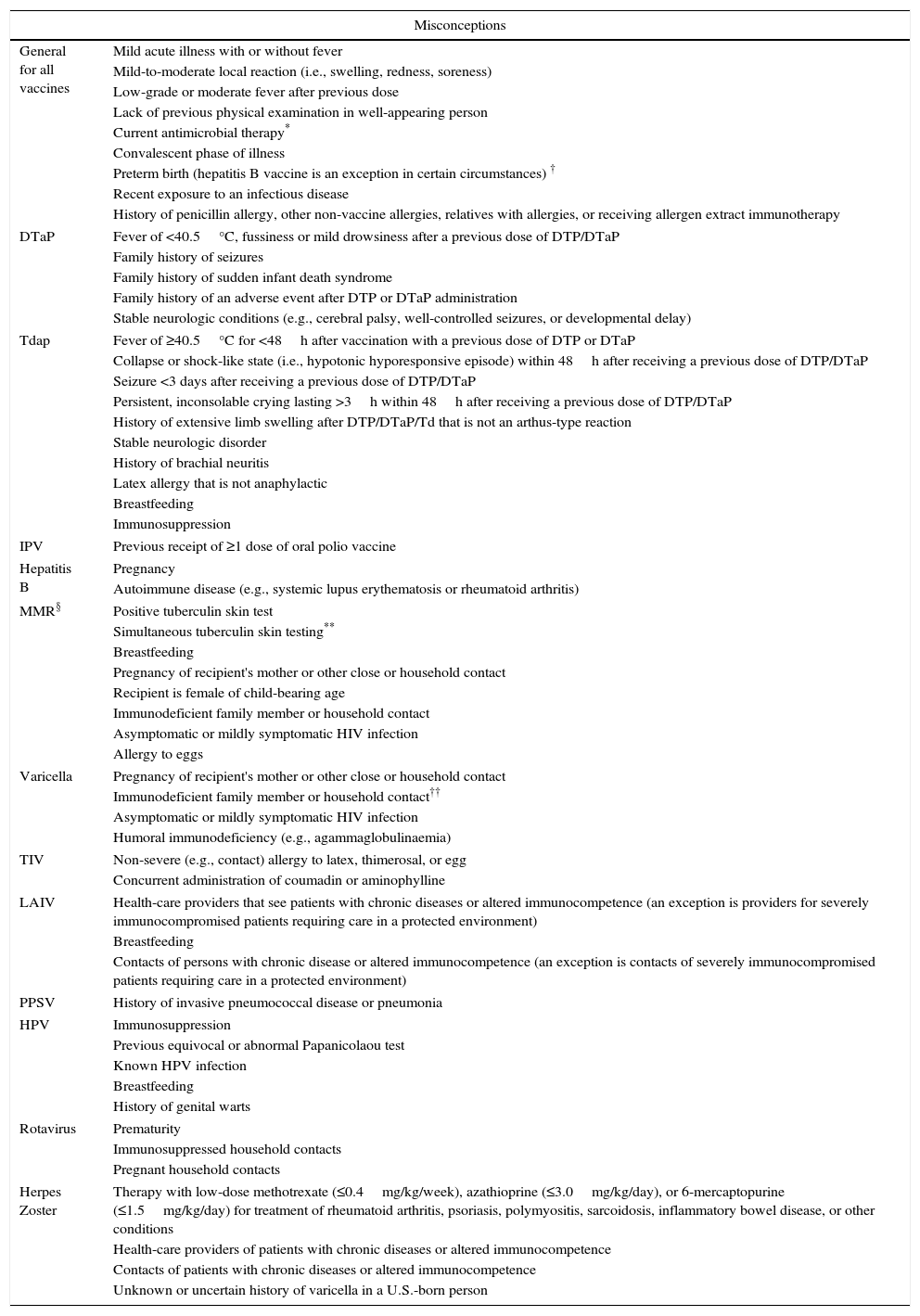
Misconceptions are conditions erroneously perceived as contraindications, although they do not imply an increased risk due to vaccination. Table 4 reports examples of misconceptions. When a personal history of disease is reported, a detailed medical history is required in order to distinguish false contraindications from hidden, true contraindications or conditions deserving additional precautions. In general, a family history of diseases or adverse events to vaccines are not considered contraindications in healthy subjects,3 but family history should always be investigated for specific conditions which need precautions (e.g. if febrile seizures occurred in relatives, separation of measles-mumps-rubella vaccine from varicella vaccine is indicated).
False contraindications. Modified from: Ref. [4].
| Misconceptions | |
|---|---|
| General for all vaccines | Mild acute illness with or without fever |
| Mild-to-moderate local reaction (i.e., swelling, redness, soreness) | |
| Low-grade or moderate fever after previous dose | |
| Lack of previous physical examination in well-appearing person | |
| Current antimicrobial therapy* | |
| Convalescent phase of illness | |
| Preterm birth (hepatitis B vaccine is an exception in certain circumstances) † | |
| Recent exposure to an infectious disease | |
| History of penicillin allergy, other non-vaccine allergies, relatives with allergies, or receiving allergen extract immunotherapy | |
| DTaP | Fever of <40.5°C, fussiness or mild drowsiness after a previous dose of DTP/DTaP |
| Family history of seizures | |
| Family history of sudden infant death syndrome | |
| Family history of an adverse event after DTP or DTaP administration | |
| Stable neurologic conditions (e.g., cerebral palsy, well-controlled seizures, or developmental delay) | |
| Tdap | Fever of ≥40.5°C for <48h after vaccination with a previous dose of DTP or DTaP |
| Collapse or shock-like state (i.e., hypotonic hyporesponsive episode) within 48h after receiving a previous dose of DTP/DTaP | |
| Seizure <3 days after receiving a previous dose of DTP/DTaP | |
| Persistent, inconsolable crying lasting >3h within 48h after receiving a previous dose of DTP/DTaP | |
| History of extensive limb swelling after DTP/DTaP/Td that is not an arthus-type reaction | |
| Stable neurologic disorder | |
| History of brachial neuritis | |
| Latex allergy that is not anaphylactic | |
| Breastfeeding | |
| Immunosuppression | |
| IPV | Previous receipt of ≥1 dose of oral polio vaccine |
| Hepatitis B | Pregnancy |
| Autoimmune disease (e.g., systemic lupus erythematosis or rheumatoid arthritis) | |
| MMR§ | Positive tuberculin skin test |
| Simultaneous tuberculin skin testing** | |
| Breastfeeding | |
| Pregnancy of recipient's mother or other close or household contact | |
| Recipient is female of child-bearing age | |
| Immunodeficient family member or household contact | |
| Asymptomatic or mildly symptomatic HIV infection | |
| Allergy to eggs | |
| Varicella | Pregnancy of recipient's mother or other close or household contact |
| Immunodeficient family member or household contact†† | |
| Asymptomatic or mildly symptomatic HIV infection | |
| Humoral immunodeficiency (e.g., agammaglobulinaemia) | |
| TIV | Non-severe (e.g., contact) allergy to latex, thimerosal, or egg |
| Concurrent administration of coumadin or aminophylline | |
| LAIV | Health-care providers that see patients with chronic diseases or altered immunocompetence (an exception is providers for severely immunocompromised patients requiring care in a protected environment) |
| Breastfeeding | |
| Contacts of persons with chronic disease or altered immunocompetence (an exception is contacts of severely immunocompromised patients requiring care in a protected environment) | |
| PPSV | History of invasive pneumococcal disease or pneumonia |
| HPV | Immunosuppression |
| Previous equivocal or abnormal Papanicolaou test | |
| Known HPV infection | |
| Breastfeeding | |
| History of genital warts | |
| Rotavirus | Prematurity |
| Immunosuppressed household contacts | |
| Pregnant household contacts | |
| Herpes Zoster | Therapy with low-dose methotrexate (≤0.4mg/kg/week), azathioprine (≤3.0mg/kg/day), or 6-mercaptopurine (≤1.5mg/kg/day) for treatment of rheumatoid arthritis, psoriasis, polymyositis, sarcoidosis, inflammatory bowel disease, or other conditions |
| Health-care providers of patients with chronic diseases or altered immunocompetence | |
| Contacts of patients with chronic diseases or altered immunocompetence | |
| Unknown or uncertain history of varicella in a U.S.-born person | |
DT, diphtheria and tetanus toxoids; DTP, diphtheria toxoidtetanus toxoid, and pertussis; DTaP, diphtheria and tetanus toxoids and acellular pertussis; HBsAg, hepatitis B surface antigen; Hib, Haemophilus influenzae type b; HPV, human papillomavirus; IPV, inactivated poliovirus; LAIV, liveattenuated influenza vaccine; MCV4, quadrivalent meningococcal conjugate vaccine; MMR, measlesmumps, and rubella; MPSV4, quadrivalent meningococcal polysaccharide vaccine; PCV, pneumococcal conjugate vaccine; PPSV, pneumococcal polysaccharide vaccine; Td, tetanus and diphtheria toxoids; Tdap, tetanus toxoidreduced diphtheria toxoid, and acellular pertussis; TIV, trivalent inactivated influenza vaccine.
Antibacterial drugs might interfere with Ty21a oral typhoid vaccine and certain antiviral drugs might interfere with varicella-containing vaccines and LAIV.
Hepatitis B vaccination should be deferred for infants weighing <2000g if the mother is documented to be HBsAg-negative at the time of the infant's birth. Vaccination can commence at chronological age one month or at hospital discharge. For infants born to HBsAg-positive women, hepatitis B immune globulin and hepatitis B vaccine should be administered within 12h after birth, regardless of weight.
MMR and varicella vaccines can be administered on the same day. If not administered on the same day, these vaccines should be separated by at least 28 days. HIV-infected children should receive immune globulin after exposure to measles. HIV-infected children can receive varicella and measles vaccine if CD4+ T-lymphocyte count is >15%. (Source: Adapted from American Academy of Pediatrics. Passive immunisation. In: Pickering LK, ed. Red book: 2009 report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2009.)
Measles vaccination might suppress tuberculin reactivity temporarily. Measles-containing vaccine can be administered on the same day as tuberculin skin testing. If testing cannot be performed until after the day of MMR vaccination, the test should be postponed for at least 4 weeks after the vaccination. If an urgent need exists to skin test, do so with the understanding that reactivity might be reduced by the vaccine.
Precautions are measures specifically adopted to rapidly identify, and properly treat, any predictable adverse events after vaccination, thereby decreasing the risks of these events evolving into serious ones.
A delay in vaccine administration is recommended in subjects with ongoing diseases who are likely to recover soon (e.g. infections), in order not to attribute symptoms incorrectly to the provided vaccination, which are really due to diseases. Whenever possible, completing an ongoing diagnostic work-up for suspected contraindications before the vaccine administration is also recommended. Indeed, the risk/benefit assessment is a priority in the decision-making in every single case.
The following situations are commonly encountered in clinical practice.
Preterm birth represents a false contraindication to vaccinations for children in stable conditions; however, associated disorders (e.g. cardiac diseases, bronchopulmonary dysplasia, neurologic disorders, recurrent infections, apnoea, chronic drug therapies…) may require precautions and a specialised pre-vaccination consultation.13–15 Similarly, patients with a non-progressive neurological disease should be evaluated for any concomitant disorders (e.g. cardiac diseases, immunodeficiency, autoimmune diseases, epilepsy…), possibly requiring additional precautions or personalised vaccine schedules.10,16,17Allergy may represent an issue: a personal history of allergy to substances not included in that specific vaccine, or well-controlled asthma are clearly misconceptions. Conversely, a personal history of hypersensitivity to vaccine components requires an evaluation of the kind of manifestation, usually cutaneous, or the time interval between allergen administration and symptom onset, in order to discriminate between immediate (likely IgE-mediated) and delayed reactions (non IgE-mediated).
Depending on the clinical features of vaccinees, examples of precautions which may be adopted are: an immunological evaluation before administration of live vaccines in subjects with recurrent infections, or complex malformative syndromes; body temperature monitoring after vaccination in subjects with a personal history of seizures induced or augmented by fever; the monitoring of pulse oximetry in periods at risk for apnoea in babies with personal history of apnoea, or very preterm birth, low birth weight and respiratory distress in the perinatal period; programming vaccine administration according to the phase of any underlying diseases, as well as the type, dose and pharmacokinetics of drugs in long-term treatments. When IgE-mediated reactions to vaccines occur, a specialised consultation is recommended (see above). It is to be remembered that indications and contraindications may differ depending on kinds of vaccines and vaccine products, since amounts of allergens may differ. In case of egg protein allergy, measles-mumps-rubella vaccine and flu vaccine can be routinely administered without preliminary testing. A suggested precaution is to keep the patient under observation for at least 30min (only in case of anaphylaxis after egg protein ingestion).9
Delayed hypersensitivity reactions to vaccine components (i.e. injection site reactions, dermatitis) are not contraindications to vaccination.9
ConclusionsVaccines are biological products that are mainly offered to healthy individuals. Therefore, the highest standards of safety are guaranteed in every step of production and marketing. Nevertheless, none of these are completely devoid of adverse events, and efficacy cannot be assured in all cases. Furthermore, while benefits of vaccination extend from the single individual to the whole society, the single individual is specifically concerned about risks of serious adverse events.
Health-care providers should be aware that potentially increased risks, as well as rare true contraindications to vaccination, exist in selected cases where specialised evaluation may be needed. Vaccine personnel should also be trained to recognise conditions that are mistakenly considered as contraindications, and to discuss real or perceived risks of adverse reactions in order to avoid missed vaccinations due to unfounded fears.
Competing interestsThe authors declare that they have no competing interests.
Authors’ contributionsThe authors are components of the Committee for Drug and Vaccine Allergies of the Italian Society for Paediatric Allergy and Immunology (SIAIP).
All authors have equally collaborated in the design and drafting of this review. All authors read and approved the final manuscript.
Authors are indebted to Dr. Andrew Tenore for editing the English version of the manuscript. We thank our colleague Roberto Bernardini for fruitful discussions and a critical revision of the manuscript.