Food allergy is a pathological immune reaction that identifies certain harmless food proteins, usually tolerated by the majority of the people, as a threat. The prevalence of these food allergies is increasing worldwide and currently affects 8% of children. Exacerbated reactions to milk, egg and peanut are the most frequent in the pediatric population. It is well known that allergic diseases are a type 2 T-helper (Th2) immune response, characterized by the elevated production of IgE antibodies. However, little is known about the immune mechanisms responsible for the development of clinical tolerance toward food allergens. Recent studies have suggested the key role of regulatory T cells (Tregs) in controlling allergic inflammation. In this review, we discuss the importance of Tregs in the pathogenesis of food allergy and the acquisition of oral tolerance in children. Further investigation in this area will be crucial for the identification of predictive markers and the development of new therapies, which will represent a clinical and social benefit for these allergic diseases.
The immune system plays the important role of distinguishing between harmful pathogens and innocuous exogenous antigens or self-antigens. Adequate immune responses react against potentially dangerous antigens, while maintaining tolerance through inoffensive antigens. Immunological tolerance implies the unresponsiveness to self-antigens and harmless antigens, and compromises both central and peripheral tolerance.1 Central tolerance occurs during T and B lymphocytes maturation in the thymus and bone marrow, respectively, when immature lymphocytes that recognize self-antigens are eliminated. Otherwise, peripheral tolerance takes place once mature lymphocytes encounter self-antigens or innocuous exogenous antigens in peripheral tissues resulting in deletion, anergy or suppression. A failure in the immunological tolerance can make the immune system recognize certain harmless antigens as a threat, leading to allergies.
Food allergy is an adverse reaction of the immune system that occurs repeatedly on exposure to particular food or food compounds, usually tolerated by the majority of the people. The prevalence of these allergic diseases worldwide is increasing dramatically in the last years. It is estimated that food allergy affects nearly 8% of children,2 which is the most frequent reason for anaphylactic reactions at this age.3 The diagnosis of food allergy is based on patient's clinical history together with total and allergen-specific serum IgE (sIgE) quantification and skin prick tests (SPTs).4,5 However, the definitive method for the confirmation of food allergy diagnosis is the Oral Food Challenge (OFC), which consists in the administration of increasing amounts of particular food allergens until any signs of an allergic reaction are perceived.6 On the other hand, OFC also carries risks depending on the patient and the doses of food administered, thus its procedure should be monitored by health specialists. Among food allergies, the inflammatory reactions related to the ingestion of cow's milk, egg, peanut and tree nuts are the most common in infants during the first years of life.7 Variation in dietary habits between countries may affect the prevalence of allergy to different foods.8 In contrast to adults, food allergies acquired in the childhood are frequently outgrown. Allergy to cow's milk and egg resolve in 50% of children by 5–6 years of age.9 However, allergy to peanut and tree nuts persists during life in 80% of the cases.10
Although food allergy represents a major public health problem in the pediatric population, little is known about the immune factors responsible of the onset of food allergies, and what the immune mechanisms are that make it possible to acquire permanent tolerance. Control of food allergies consists in food ingestion avoidance4 or treatment of the symptoms derived from adverse reactions.11 A better understanding of the immune alterations that develop the differential factors between allergy and tolerance is crucial for the prevention, the diagnosis and the treatment of these food allergies. This review highlights up-to-date advances in the discovery of new immune cell populations, which are known to be implicated in food allergy and oral tolerance, focusing on the role of regulatory T cells in children.
Immune mechanisms implicated in the development of allergyAllergic diseases are well known to be a type 2 immune response characterized by increased numbers of type 2 T-helper (Th2) cells and type 2 innate lymphoid (ILC2s) cells that secrete interleukin-4 (IL-4), IL-5 and IL-13. Beyond the classical Th2 cells, other recently identified effector T-cell subsets, including Th9, Th17 and Th22, along with Th2 cytokines such as IL-9, IL-25, IL-31 and IL-33, might also be participating in allergic diseases.12,13 These cells play a pivotal role in the activation and recruitment of basophils, mast cells and eosinophils to the affected tissues, together with the production of allergen-specific IgE antibodies.14
The immune mechanism implicated in food allergy comprises two main stages: a first sensitization phase and a second effector phase. Food allergens are exposed to the immune system through different mechanisms. The sensitization mainly occurs in the gastrointestinal tract, but also in the skin and the respiratory tract later to the first contact with particular ingested food allergens. While intestinal sensitization can cause type I food allergy, epicutaneous and respiratory sensitization can cause type II and type III food allergy, respectively. This sensitization phase involves the generation and expansion of allergen-specific Th2 cells and the production of specific IgE antibodies that bind to the Fc¿RI on the surface of mast cells and basophils. After subsequent exposures to the originative food allergens and during the effector phase, allergens crosslink to IgE-Fc¿RI on effector cells triggering their activation and the release of inflammatory mediators, such as histamine, contributing to the allergic inflammation characteristic symptoms.15
Even if Th2 immunity is considered the major mediator in the development of allergy, little is known about which immune mechanism or alterations are responsible for triggering this inflammatory cascade that produce the onset of an allergy. Considering that Th2 cells are present in the exposed tissues of any subject, why do only some children develop food allergy whilst others do not? Which immune alteration constitutes the differential factor between developing allergy or tolerance remains unclear, and this question needs to be elucidated to improve the prevention, the diagnosis and the treatment of these food allergies.
Oral tolerance to food allergens: regulatory T cellsOral tolerance is a type of peripheral tolerance that implies the non-responsiveness to ingested food proteins.16 Physiologically, the role of oral tolerance is to prevent activation of lymphocytes specific for innocuous food antigens. Disturbance in the aforementioned oral tolerance may lead to food allergy and therefore, the induction of tolerance could solve the development of these allergic diseases.17 Deletion, anergy and ignorance are mechanisms that the immune system employs to maintain oral tolerance through cell death or functional unresponsiveness of lymphocytes capable of responding to food antigens. Deletion consists in cell death by apoptosis, while anergy is the induction of a permanent state of non-response to the antigen in which cells do not die; lastly, ignorance is due to the inability to detect the antigen by cells with affinity for that antigen. In addition, there are other tolerance mechanisms that operate through the generation of regulatory T cells (Treg) in which we will focus on.
Treg cells are a specialized subpopulation of CD4+ T cells that play a crucial role in the establishment and maintenance of immunological homeostasis.18 These Tregs have the capacity to suppress the effector function of a wide range of cells, including Th2 cells, antibody-producing B cells, and other immune subsets,19 and then Tregs have the capacity to prevent inadequate immune responses such as allergic processes. Treg cells are largely classified into natural Treg (nTreg) cells, produced in the thymus in response to self-antigens, and inducible Treg (iTreg) cells, generated in the periphery from naïve CD4+ T cells. nTregs are identified as CD4+CD25+Foxp3+ cells together with the production of IL-10 and Transforming Growth Factor-β (TGF-β). Additionally, three different iTregs subsets have been described: (i) induced Foxp3+ Treg cells, (ii) type 1 Treg (Tr1) cells and (iii) Th3 cells. Unlike Foxp3+ iTregs, Tr1 and Th3 cells do not express the master regulator transcription factor Foxp3. However, Tr1 cells are characterized to coexpress CD49b and LAG-3 surface markers and secrete high levels of IL-1020 while Th3 cells are defined by the expression of LAP and the production of TGF-β.21,22 In this review, we will focus on the better-established and defined Foxp3+ Treg cell population.
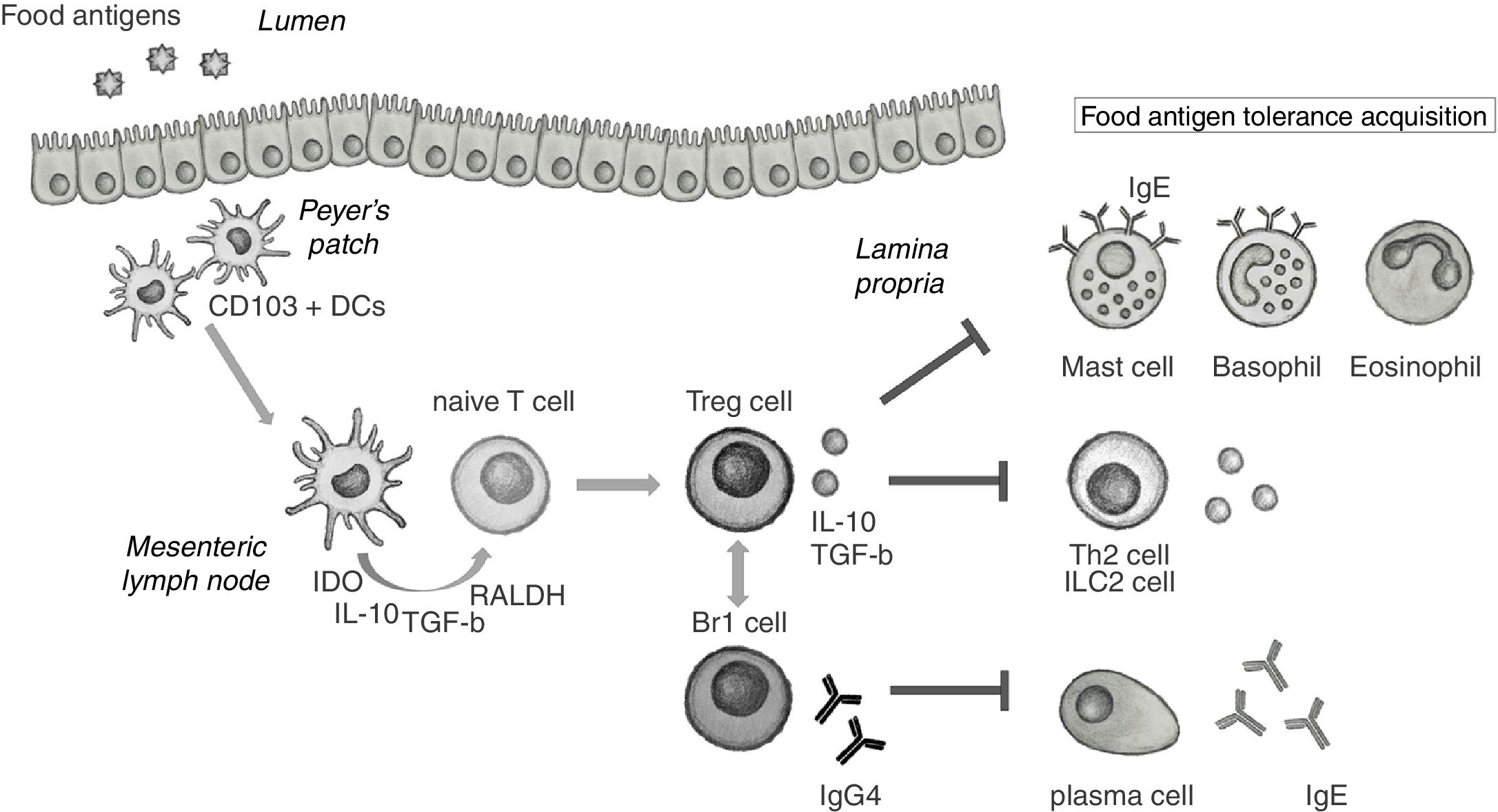
It is known that during local inflammation, food allergen-specific Tregs are produced in the gastrointestinal tract.23 The generation of Treg cells in the gut and gut-associated lymphoid tissue (GALT) needs from specific mucosal dendritic cells (DCs).24 Food antigens are uptaken from the lumen by CD103+ DCs in Peyer's patches (PP) and migrate to the mesenteric lymph nodes (mLN). In the mLN, CD103+ DCs secrete high levels of indoleamine 2,3-dioxygenase (IDO), IL-10, TGF-β and RALDH, responsible for the production of retinoic acid (RA). All these signals promote the differentiation of allergen-specific Treg cells from naïve T cells25 that migrate back to the lamina propria to achieve their suppressive functions (Fig. 1). It has been contemplated that part of these Tregs can escape the local tissue and get into the circulation, thus acting more generally in other systemic tissues.26 In addition to allergen-specific Tregs, circulating polyclonal (non-specific) Tregs could also play a suppressive role in food allergy through the production of anti-inflammatory cytokines and be a marker of patient's intrinsic tolerance.
Role of regulatory cells in the acquisition of tolerance toward food antigens in the gastrointestinal mucosa. CD103+ DCs in Peyer's patches uptake food antigens from the lumen and migrate to the mesenteric lymph nodes (mLN). In the mLN, DCs secrete indoleamine 2,3-dioxygenase (IDO), IL-10, TGF-beta and retinaldehyde dehydrogenase (RALDH) that induce naïve T cells to differentiate into Treg cells. Once in the lamina propria, Treg cells are able to suppress the activation of effector cells implicated in food allergic inflammation, such as mast cells, basophils and eosinophils. Treg also suppress Th2 and ILC2 cells and their secretion of IL-4, IL-5 and IL-13, and participate in the induction of IgG4 by Br1 cells that block IgE activity.
Treg cells are able to control the sensitization and effector phases of food allergic reactions through several suppression mechanisms. These mechanisms include secretion of inhibitory cytokines, such as IL-10 and TGF-β; cytolysis induced by granzymes; metabolic deprivation, as IL-2 consumption; and down-modulation of antigen-presenting cells (APC).23 Tregs act directly and indirectly on many cells implicated in responses to food antigens that come upon the GALT. Treg cells suppress effector T cell activation and cytokine secretion, mast cell and basophil degranulation27 and eosinophil and T cell infiltration to inflamed tissues.28 Tregs also participate in the production of allergen-specific IgG4 by B cells, a non-inflammatory immunoglobulin that blocks IgE activity.29,30 In addition, Tregs are involved in the generation of tolerogenic DC31 and the interaction with injured tissue provoking tissue repair.32
Apart from regulatory T cells, there are other cells implicated in oral tolerance acquisition. Regulatory B cells (Breg) are immunosuppressive B cells, characterized by the production of IL-10, IL-35 and TGF-β. Through the secretion of these anti-inflammatory cytokines, Breg cells suppress DC maturation, thus suppressing the activation and proliferation of effector T cells, and also induce the differentiation of Foxp3+ Treg cells and Tr1 cells.33 IL-10 secreting Breg (Br1) cells, a subtype of Bregs, have been deeply studied in tolerance induction for being the main producers of IgG4, as well.34 In addition to Treg cells and Breg cells, other cell subsets with suppressive capacity have been described.35 IL-10 producing DCs,36 IL-10 producing NK cells,37 CD8+ T cells,38 γδ T cells39 and some macrophages40 subsets may also participate in the control of excessive immune responses. However, for instance the implication of CD8+ T cell in oral tolerance needs to be clarified.41
Regulatory T cells in food allergic diseasesTregs have gained a major role in recent years given their involvement in transplant tolerance, autoimmune and allergic diseases42 and also due to their potential clinical use in the treatment of immunological diseases and in the physiological and pathological control of immune responses.43
In mouse models, it has been proved that proliferation of Tregs in the gut is required to achieve intestinal tolerance.44 Besides, adaptive transfer of Tregs has shown a protective effect on allergic inflammation, inducing immune tolerance to certain allergens.45 Moreover, Foxp3-deficient mice lead to severe lymphoproliferation and inappropriate control of food antigen uptake, characterized by elevated IgE levels and eosinophilia.46 In humans, mutations in Foxp3 locus cause immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. This syndrome has been associated to inducing pronounced allergic inflammation in patients, due to a failure in the functional capacity of Tregs.47 In addition, iTreg cells generated in periphery have been demonstrated to play an essential role rather than nTregs in controlling mucosal Th2 inflammation.48
Definitely, Treg cells are required to induce tolerance to food allergens encountered in GALT and allergic inflammation is related to an impairment or deregulation of Tregs. For instance, it has been demonstrated that ILC2, through the production of large amounts of IL-4, inhibit Treg cell function.49 Mast cell and IgE are also able to impair Treg induction during food allergic responses.50 Furthermore, allergen-specific Treg cells presented in a proinflammatory environment can be reprogrammed toward a “Th2 cell-like effector phenotype” aggravating the food allergy disease.51 All these mechanisms that provoke a disruption in Tregs, ultimately prevent the control in the pathogenesis of food allergy.
Immune regulation in patients with food allergyThe important role of Treg in controlling healthy immune responses to food allergies has been confirmed in patients through several studies. We have shown that both preterm and full-term infants are born with similar and even superior Treg percentages and absolute counts to those in adolescents and adults,52 given their implication in maternal-fetal tolerance. Therefore, Treg could play a central role in the maturation of the immune system and in the onset of immune-related diseases, which are frequent in the initial period of life. The immaturity but also the plasticity of the immune system in children, together with their preserved thymic functionality, which is the source of nTreg cells, could be determinant in both, the onset of food allergies and the acquisition of tolerance or desensitization to allergens.
Akdis et al. have demonstrated that higher frequencies of allergen-specific Treg cells were encountered in healthy adult patients, whereas elevated frequencies of Th2 effector T cells were observed in allergic adult patients. In addition, Tregs isolated from the blood of allergic patients were showed to be less functional than Tregs purified from healthy subjects.53 Likewise, food-allergic infants showed impaired Treg responses in comparison to non-allergic infants following in vivo exposure to egg or peanut allergens. Dang et al. also demonstrated that these food-allergic infants have a lower Treg:Teff ratio than healthy patients.54 In our group, we have investigated the differences in the immune system between food-allergic children and non-allergic controls. Our results demonstrated the presence of an increased frequency of IL4-secreting CD4+ T cells associated with a deficit in the number of Treg cells in children with cow's milk protein allergy (CMPA),55 and also an increased number of Th2 effector cells associated with a deficit of Treg in egg-allergic children.56 We also found that the ratio Treg:IL4-Th2 was reduced in CMPA children,55 and the ratio Treg:Th2-effector was significantly reduced in egg-allergic children.56 Therefore, the balance between Th2 effector T cells and Treg cells is what lastly determines either the development of an allergic response (Th2) or a tolerance (Treg) toward allergens.57
The majority of the studies performed in allergy are carried out with already established allergic patients. However, in this context it is complicated to discern whether a previous impairment of Treg could be the cause of the allergy onset. Thus, we have recently analyzed the Treg values in cow's milk protein allergic infants with suspicion of CMPA during the period of 1–3 days after their first adverse reaction.55 Our study demonstrated that infants with decreased Treg values were just the patients that were subsequently diagnosed as CMPA by a provocation test. Another study has demonstrated that newborns born with decreased functional Tregs were related to a subsequent egg allergy development during childhood.58 This reinforces our idea that a deficit in the Treg population could predispose to the acquisition of food allergy in infants.
Going through the mechanisms that could explain the deficit of Tregs in allergic infants, our group observed that Treg deficit in CMPA infants was correlated with decreased serum levels of vitamin D, and both Treg and Vitamin D deficit were good predictors of CMPA diagnosis. Interestingly basal levels of Vitamin D also predicted those CMPA patients developing spontaneous tolerance in the first year.55 Besides, a direct correlation between plasma levels of vitamin D and Treg counts was observed.55 In fact, 25-hidroxyvitamin D has denoted immunoregulatory properties and the capacity to induce and preserve Treg cells in humans.59,60 Moreover, several studies have associated vitamin D insufficiency with not only a higher incidence of food allergy in infants61–64 but also a higher susceptibility to gastrointestinal infections owing to affecting the epithelial barrier integrity.65 Thus, we hypothesized that after cow's milk protein ingestion, children with a deficit in serum vitamin D levels may have an impaired survival of Treg cells subsequently leading to a prevalence of the Th2 effector response along with allergic manifestations.55 However, there are studies that show the controversy that exists when relating low levels of vitamin D with a greater susceptibility of diseases that befall with high levels of IgE as shown by the Mendelian randomization study of Manousaky et al.66 These findings query the use of vitamin D supplementation for the prevention of elevated IgE levels and suggest further studies to validate the relationship between vitamin D and the onset of allergic diseases.
Regulatory T cells play a major role in the success of specific oral tolerance induction (SOTI) protocols to food allergensRegarding the role of Treg in the induction of tolerance to allergens, Karlsson et al.67 showed that children who outgrew their cow's milk allergy presented higher frequencies of circulating Tregs and reduced in vitro proliferative responses to specific milk allergens in comparison to still allergic children. Moreover, higher frequencies of allergen-specific Tregs have been correlated with a mild clinical phenotype and a favorable prognosis in milk allergic children.68 Although there are currently no treatments to induce definitive tolerance, specific oral tolerance induction (SOTI), also named oral immunotherapy (OIT) is able to achieve gradual desensitization in food allergic patients through repeated exposures to increasing doses of the causative allergen.69 Clinical trials performed with milk, egg and peanut allergic children showed that OIT is the most encouraging desensitization method proven to be safe and effective for the treatment of food allergy.70–74 Its mechanism of action seems to occur in the gastrointestinal tract specifically on mucosal DCs, thus affecting the production of Treg cells.75 Furthermore, the success of OIT comprises the generation and maintenance of functional allergen-specific Tregs, changes in Treg:Teff ratio, increased levels of serum allergen-specific IgG4 and decreased basophil and mast cell reactivity.71,76 Previous studies in our group revealed that desensitization of hen's egg allergic children after SOTI was accompanied by decreased effector-memory CD4+ T cells along with a marked increase of a particular subset of CD4+ T cells, defined as CD4+CD38+CD45RO- with hypo-proliferative and non-reactive properties, which may show a switch in CD4+ T cells from an active to a hypo-proliferative phenotype.77 We also demonstrate that children who acquired tolerance after SOTI experienced an increase in the Treg population, reaching values even higher than those observed in non-allergic children of similar ages. This marked increase in Tregs was one of the factors associated with the decrease of effector T cells and desensitization, reflecting once again the potential of the Treg cell population to control inadequate immune responses in children.56
ConclusionsAllergic diseases are characterized to be a Th2 immune response. However, in recent years, a great deal of progress has been made in the discovery of new immune populations, apart from Th2 cells, that play an important role in regulating the immune response to food allergens. Treg cells are a crucial population for the establishment and maintenance of immunological homeostasis, being able to control the sensitization and effector phases of food allergic reactions. Impaired Treg responses have been shown in food allergic children, while higher frequencies of Tregs have been found in healthy children and those who achieved tolerance. The deficit of Treg seems to be a factor predisposing for the food-allergy onset in infants, and also Treg cells would play a crucial role in desensitization and the acquisition of tolerance to food allergens. Thus, Treg cell counts and vitamin D levels could be predictive markers of food allergy in children, hence avoiding the risk associated with Oral Food Challenge, and may also be used for the clinical follow-up of the patients toward tolerance induction or persistent allergy. Nevertheless, further studies in larger cohorts of children must be performed to consider the validation of these markers and the application thereof as a target for the development of new treatments for food allergic children are requested.
Sources of fundingAll the authors declare that they have no competing financial interests. This work was supported by a grant from Sociedad Española de Inmunología Clínica, Alergología y Asma Pediátrica (SEICAP) and by grants from Instituto de Salud Carlos III (ISCIII) co-financed by FEDER funds (PI15/00011; ICI14/00282). E.BDQ is supported by a grant (PEJ15/BIO/AI-0251) from the Community of Madrid through the EU program Youth Employment Initiative (YEI).
Conflict of interestThe authors have no conflict of interest to declare.
This work was supported by a grant from Sociedad Española de Inmunología Clínica, Alergología y Asma Pediátrica (SEICAP). The authors also thank Elena Blázquez López for the provided help in the hand-drawing scheme showed in Fig. 1.