Despite growing evidence suggesting potential association between innate and adaptive immunity in viral-induced acute asthma, there is paucity of data in this area.
ObjectiveThis study aimed to investigate the association of innate and adaptive immunity with acute asthma attacks by analysing the role of IFN-γ-inducible protein 10 (IP-10), TLR2, cathelicidin, vitamin D and cytokines.
Material and methodsThis prospective study included 33 patients with viral-induced acute asthma and 30 children with controlled asthma. Nasopharyngeal swab samples were collected for virus identification and asthma attack scores assessed in acute asthma group. Blood sampling for IP-10, TLR2, cathelicidin, vitamin D levels, and spirometric indices were employed.
ResultsSerum IP-10 and cathelicidin levels of acute asthma group were significantly higher and vitamin D levels were lower than controlled asthma group (IP-10; p=0.006, cathelicidin; p=0.002, vitamin D; p<0.001). Serum IP-10 levels showed a significant negative correlation with age (p=0.009), TLR2 (p=0.05) and spirometric indices (p=0.002) in all asthmatics and a significant positive correlation with parameters of asthma attack severity (p=0.03) in acute asthma group. Higher cathelicidin values showed significant positive relation to IP-10 (beta coefficient: 33, p=0.02). Serum IP-10 levels higher than 38.9pg/ml (sensitivity: 85%, specificity: 47%, p=0.002) were predictive of virus-induced asthma. Serum IP-10 and vitamin D levels were found to be significantly related to viral-asthma attacks (IP-10; aOR: 8.93, p=0.03 and vitamin D; aOR: 0.82, p=0.001).
ConclusionsInnate immunity biomarkers such as serum IP-10 and cathelicidin can be used to predict viral-induced acute asthma. These biomarkers may provide potential new treatment targets for acute asthma.
Asthma exacerbations are major burdens on the healthcare system and contribute significantly to morbidity and mortality.1 Viral respiratory tract infections play an important role in asthma exacerbations.2 The pathogenesis of viral-induced asthma is poorly understood and clearly different from the pathogenesis of allergen-induced asthma.3 Understanding the mechanisms of viral-induced exacerbations would be useful in the clinical management of acute asthma attacks.
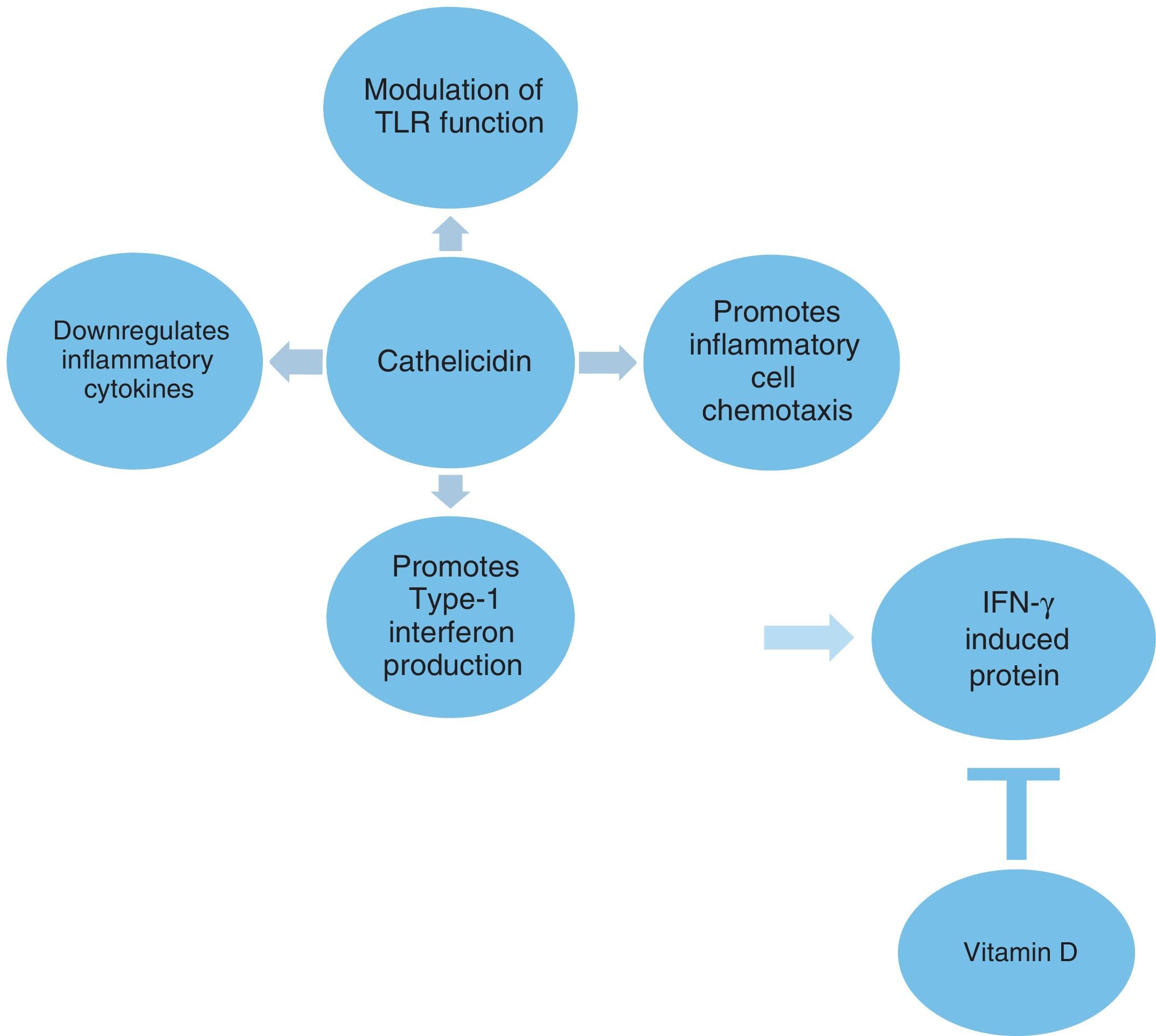
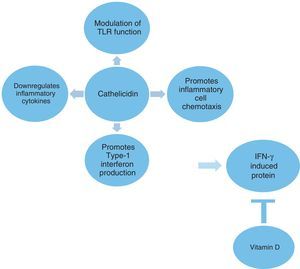
The innate immune system plays a crucial role in the defense against microbes as well as in the initiation of inflammatory responses. Cathelicidin is a multifunctional host defense molecule that has several effects on the innate and adaptive immune systems that may be relevant in the modulation of infections (Fig. 1). It has the potential to influence and modulate Toll-like receptor (TLR) mediated responses and the activity of various cell populations involved in inflammatory processes. Cathelicidins also promote the production of type 1 interferons, which are the important part of the innate immune response to microbes.4,5 The role of cathelicidins in viral-induced asthma exacerbations is not well known.
The inflammatory process in asthma, including multiple cell types and mediators, is quite complex.6 The activation of TLRs, which have been shown to play an important role in pathogen recognition, leads to signal transduction involving a range of cytokines and chemokines.7 IFN-gamma-inducible protein 10 (IP-10) is a Th1-type chemokine that is involved in both innate and adaptive immunity. It is secreted from cells stimulated with type I and II IFNs and lipopolysaccharide.8,9 The expression of IP-10 is observed in many Th1-type inflammatory diseases, in which it is considered to play an important role in recruiting activated T cells into sites of tissue inflammation.10,11 It has also been shown to be increased in the airways of asthmatics.8,10 However, its regulation in Th2-type inflammation and viral-induced asthma still remains elusive. To date, no study has yet evaluated the association between IP-10 and cathelicidin in viral-induced acute asthma.
Vitamin D (vit D) has several effects on the innate and adaptive immune systems that may be relevant in the modulation of the severity of asthma attacks.12 Previous studies showed that vit D inhibited IP-10 secretion in a concentration-dependent manner and increased the transcription of the innate immune protein, human cathelicidin antimicrobial peptide.13,14
Despite the accumulating evidence that suggests a potential association between innate and adaptive immunity in viral-induced acute asthma, data on this area is scarce. The aim of the study was to investigate the underlying mechanisms of viral-induced asthma exacerbations and to determine the association between innate and adaptive immunity in acute asthma by analysing the role of IP-10, cathelicidin, TLR and cytokines.
Material and methodsAsthmatic patients admitted to the pediatric allergy outpatient clinic for an asthma exacerbation or a routine control visit between November 2011 and February 2012 were included in this study. Classification of asthma severity and control was assessed according to the criteria of the Global Initiative for Asthma.15 Controlled asthma was defined as the absence of nocturnal and daytime symptoms, limitation of activities, need for rescue treatment with normal lung function tests for the last month. Asthma attack was defined as the presence of progressive cough, wheezing, dyspnea or chest tightness accompanied by low forced expiratory volume in 1 s (FEV1) values in a previously diagnosed asthmatic patient. The asthma attack score, pulmonary index score, need for systemic steroid, hospitalization and number of bronchodilators in the first 6h of attack were also assessed for the patients in the acute asthma group.16,17 Regular medications were recorded in all patients. The asthma attack and controlled asthma groups were matched by mild to moderate severity in the previous three months and they were monosensitised to house dust mites. The inclusion criteria were as follows: (1) presence of symptoms of upper respiratory tract infection (URTI) in the acute asthma group; and (2) no symptoms of URTI in the preceding six weeks in the controlled asthma group. The exclusion criteria were as follows: (1) presence of chronic lung disease or severe asthma; (2) sensitization to allergen other than mite; and (3) use of drugs or a chronic disease that can affect the levels of serum vit D.
The children were asked about the presence of symptoms of URTI. The criteria for a clinically manifested URTI were defined as two or more of the following symptoms: fever, cough, headache, sneezing/runny nose/nasal congestion, pharyngeal hyperemia and sore throat.2
Lung functions were conducted just before blood sampling, in accordance with ATS/ERS guidelines18 using a spirometry device (Jaeger, Germany). The values of FEV1, forced vital capacity (FVC), peak expiratory flow (PEF), maximum midexpiratory flow (MMEF) and bronchodilator reversibility were recorded for data analysis.
Allergen sensitization was evaluated by skin testing. It was performed according to the ISAAC protocol.19 The following antigens were applied to the volar surface of the forearm in addition to histamine and saline controls: Dermatophagoides pteronyssinus, Dermatophagoides farinae, cockroach, cat and dog dander, Alternaria alternata, mixed grass and tree pollen. A test was considered positive if the maximal diameter of the wheal was 3mm after subtracting the maximal diameter of the negative control.
Body mass index (BMI) was calculated as weight in kilograms divided by the height in squared meters and was recorded.
The levels of serum IP-10 (RayBiotech Inc., Norcross, USA), human cathelicidin antimicrobial peptide (camp) (USCN Life Science Inc., Houston, Texas, USA) and TLR2 (Cusabio Biotech Co. Ltd., Wuhan, China) were measured by ELISA using an enzyme-linked immunosorbent assay. Serum 25-hydroxyvitamin D levels were analyzed by high-performance liquid chromatography system tandem mass spectrometry (Chromsystems, Grafelfing, Germany). Moreover, the total IgE levels and eosinophil count measurements were performed as markers of allergy.
Cytokines such as IL-13, TGF-β (RayBiotech Inc., Norcross, USA), IL-6, IL-12, IL-10, IL-4 and IFN-γ (DIAsource ImmunoAssays S.A., Nivelles, Belgium) were measured in serum in all patients by ELISA assay. Peripheral blood mononuclear cells were isolated by Ficoll centrifugation. Lymphocytes were gated on the basis of their forward and side scatter properties using FACSCalibur flow cytometry (Becton Dickinson, New Jersey, USA). Peripheral blood CD4+, CD8+, CD25+ and Foxp3 positive cell numbers were studied and T reg cells were determined as the absolute count of CD4+CD25+Foxp3+ cells. In the asthma attack group, blood samples were taken at the time point when the patients were admitted to hospital with an asthma exacerbation.
Nasopharyngeal swab samples were collected from children with acute asthma. By using multiplex-PCR (Seegene Inc., Seoul, Korea), the nasopharyngeal swab samples were tested for 15 different viruses (rhinovirus A/B/C, respiratory syncytial virus, enteroviruses, coronaviruses 229E/NL63 and OC43/HKU1, metapneumovirus, human bocavirus, influenza A and B viruses, adenovirus and parainfluenza virus 1, 2, 3, 4).
The study was approved by the clinical research ethics committees of Mersin University. All patients provided written informed consent prior to taking part in the study.
Statistical analysisStatistical analyses were performed using the SPSS 11.5.1 statistical software for Windows. The Shapiro–Wilk test was performed to test the suitability of the normal distribution of the numerical data. Descriptive analyses were presented using median (minimum–maximum) for variables not distributed normally and means±standard deviations (SD) for normally distributed variables. Unadjusted comparisons were made using the Independent samples t-test or Mann–Whitney U test for continuous endpoints and the Chi-Square test for categorical endpoints. The logarithm of cathelicidin was calculated because of the wide range of values. Correlations were assessed using the Spearman's correlation test. The possible factors identified with univariate analysis (p<0.25) and the ones that were considered clinically important were further entered into the multiple regression analysis to determine the independent predictors. A multiple linear regression model was used to identify independent predictors of serum IP-10 levels. The risk factors that could influence the development of asthma attacks were evaluated in all asthmatics with multiple logistic analysis. Roc curve analysis was performed to determine the cut-off value for IP-10 to predict the development of a viral asthma attack. Odds ratios with their 95% confidence intervals were estimated. A p value of less than 0.05 was considered statistically significant.
ResultsA total of 63 children (33 patients with viral-induced asthma attack and 30 patients with controlled asthma) were enrolled. The mean age was 10.7±3.1 years and 22 (34.9%) of the patients were female. The mean age of the asthma attack group was significantly lower than the controlled asthma group (p<0.05). No difference was found between the two asthma groups in terms of gender, inhaled corticosteroid usage, BMI and markers of allergy (total IgE and serum eosinophils).
Among the 33 samples from patients with acute asthma, parainfluenza virus was detected in 10 (30.3%), rhinovirus in six (18.2%), RSV in six (18.2%), adenovirus in five (15.1%), influenza virus in four (12.1%), and coronavirus in two (6%).
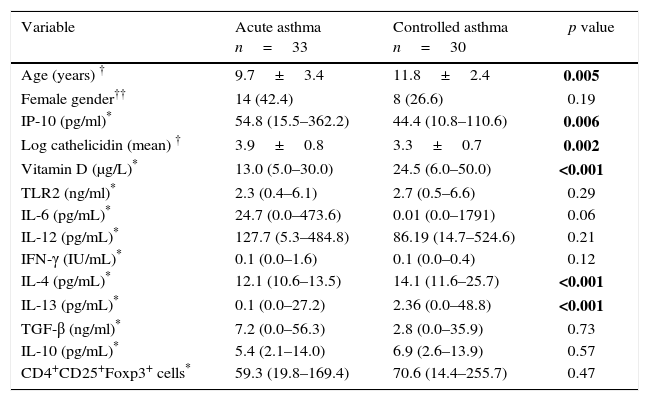
The median serum IP-10 level was 54.8pg/ml in the acute asthma group and 44.4pg/ml in the controlled asthma group. The difference between groups was significant (p=0.006). The mean log camp levels were also significantly higher in the acute asthma group than the controlled asthma group (p=0.002). The median serum vit D level was 13μg/L in acute asthma group and 24.5μg/L in the controlled asthma group. The difference between the two groups was highly significant (p<0.001). No difference was found between the groups in terms of TLR2 levels. Serum IL-4 and IL-13 levels were lower in the acute asthma group than the controlled asthma (p<0.001). There was no significant difference in terms of other cytokines and the number of Foxp3+ T reg cells (Table 1).
The comparison of demographic characteristics, serum IP-10, cathelicidin, vitamin D and cytokine levels between study groups.
| Variable | Acute asthma n=33 | Controlled asthma n=30 | p value |
|---|---|---|---|
| Age (years) † | 9.7±3.4 | 11.8±2.4 | 0.005 |
| Female gender†† | 14 (42.4) | 8 (26.6) | 0.19 |
| IP-10 (pg/ml)* | 54.8 (15.5–362.2) | 44.4 (10.8–110.6) | 0.006 |
| Log cathelicidin (mean) † | 3.9±0.8 | 3.3±0.7 | 0.002 |
| Vitamin D (μg/L)* | 13.0 (5.0–30.0) | 24.5 (6.0–50.0) | <0.001 |
| TLR2 (ng/ml)* | 2.3 (0.4–6.1) | 2.7 (0.5–6.6) | 0.29 |
| IL-6 (pg/mL)* | 24.7 (0.0–473.6) | 0.01 (0.0–1791) | 0.06 |
| IL-12 (pg/mL)* | 127.7 (5.3–484.8) | 86.19 (14.7–524.6) | 0.21 |
| IFN-γ (IU/mL)* | 0.1 (0.0–1.6) | 0.1 (0.0–0.4) | 0.12 |
| IL-4 (pg/mL)* | 12.1 (10.6–13.5) | 14.1 (11.6–25.7) | <0.001 |
| IL-13 (pg/mL)* | 0.1 (0.0–27.2) | 2.36 (0.0–48.8) | <0.001 |
| TGF-β (ng/ml)* | 7.2 (0.0–56.3) | 2.8 (0.0–35.9) | 0.73 |
| IL-10 (pg/mL)* | 5.4 (2.1–14.0) | 6.9 (2.6–13.9) | 0.57 |
| CD4+CD25+Foxp3+ cells* | 59.3 (19.8–169.4) | 70.6 (14.4–255.7) | 0.47 |
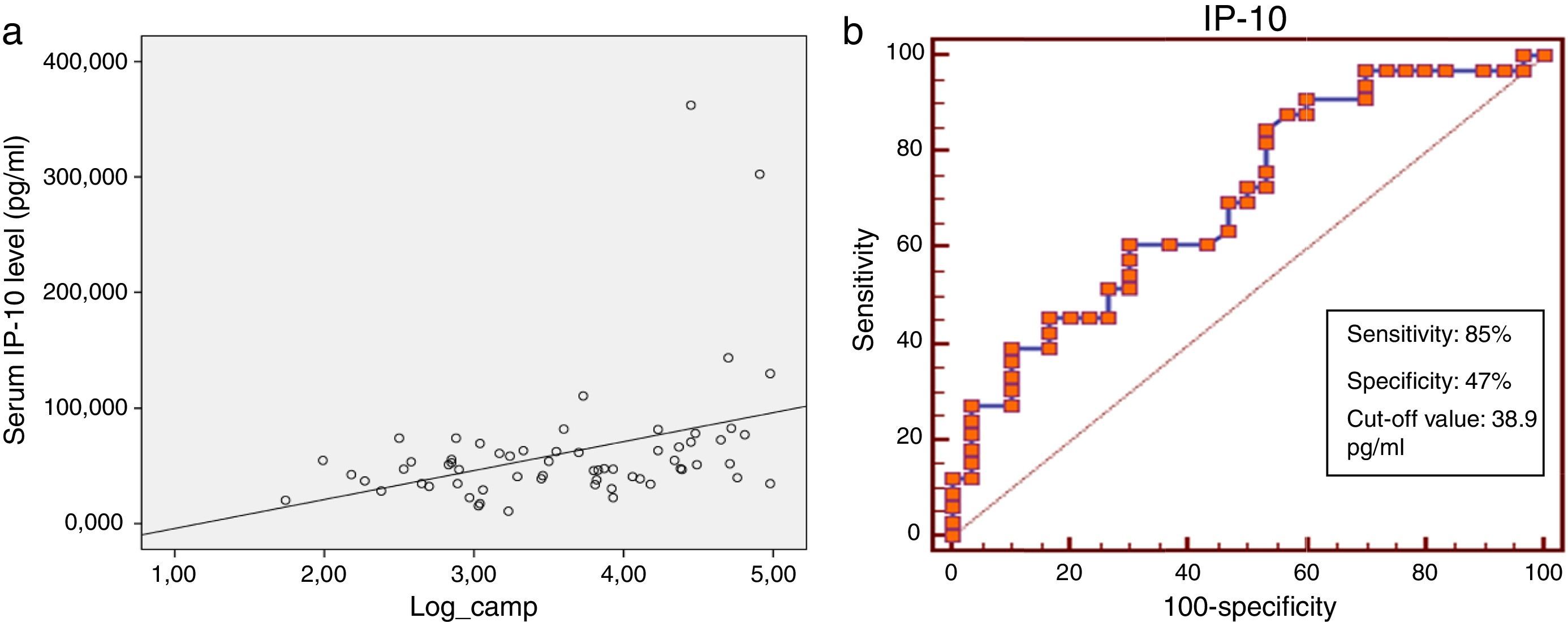
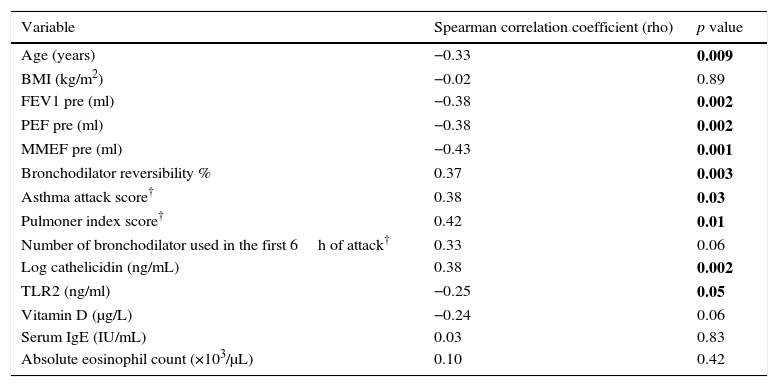
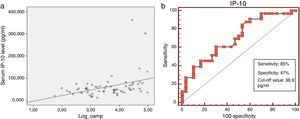
Serum IP-10 levels showed a significant negative correlation with age (Spearman correlation coefficient rho: −0.33, p=0.009) and TLR2 levels (Spearman correlation coefficient rho: −0.25, p=0.05) and a positive correlation with serum cathelicidin levels (Spearman correlation coefficient rho: 0.38, p=0.002) (Fig. 2a). A negative correlation was found between IP-10 and vit D on the border of significance (p=0.06). No significant correlation was found between IP-10 levels and gender, BMI, ICS usage, total IgE, absolute eosinophil counts, Foxp3+ T reg cells and cytokines among all asthma patients in the univariate analysis (Table 2).
Univariate analysis of associations between serum IP-10 levels and continuous clinical variables, cathelicidin, vitamin D and cytokines in asthmatics.
| Variable | Spearman correlation coefficient (rho) | p value |
|---|---|---|
| Age (years) | −0.33 | 0.009 |
| BMI (kg/m2) | −0.02 | 0.89 |
| FEV1 pre (ml) | −0.38 | 0.002 |
| PEF pre (ml) | −0.38 | 0.002 |
| MMEF pre (ml) | −0.43 | 0.001 |
| Bronchodilator reversibility % | 0.37 | 0.003 |
| Asthma attack score† | 0.38 | 0.03 |
| Pulmoner index score† | 0.42 | 0.01 |
| Number of bronchodilator used in the first 6h of attack† | 0.33 | 0.06 |
| Log cathelicidin (ng/mL) | 0.38 | 0.002 |
| TLR2 (ng/ml) | −0.25 | 0.05 |
| Vitamin D (μg/L) | −0.24 | 0.06 |
| Serum IgE (IU/mL) | 0.03 | 0.83 |
| Absolute eosinophil count (×103/μL) | 0.10 | 0.42 |
ICS, Inhaled corticosteroids; BMI, body mass index.
A significant negative correlation was found between serum IP-10 levels and the spirometric indices (FEV1 rho: −0.38, p=0.002, PEF rho: −0.38, p=0.002, MMEF rho: −0.43, p=0.001) and a positive correlation with bronchodilator reversibility (rho: 0.37, p=0.003). In the acute asthma group, a significant positive correlation was found between IP-10 and the parameters of asthma attack severity (asthma attack score rho: 0.38, p=0.03, pulmonary index score rho: 0.42, p=0.01), but the number of bronchodilator used in the first 6h of attack was found to be correlated with IP-10 on the border of significance (rho: 0.33, p=0.06) (Table 2). No correlation was found between IP-10 and the need for systemic steroid and hospitalization.
Serum cathelicidin levels showed a significant positive correlation with IL-6 (Spearman correlation coefficient rho: 0.34, p=0.006) and a negative correlation with TLR2 (Spearman correlation coefficient rho: −0.32, p=0.01), IL-4 (Spearman correlation coefficient rho: −0.3, p=0.02) and IL-13 (Spearman correlation coefficient rho: −0.34, p=0.006) levels. No significant correlation was found between cathelicidin and Foxp3+ T reg cells and other cytokines.
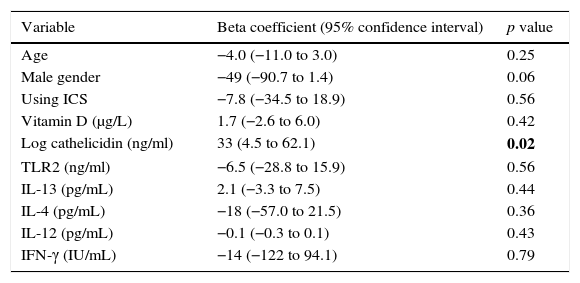
Multiple linear regression analysis of possible factors that could influence serum IP-10 levels in the acute asthma group revealed that higher cathelicidin values showed a significant positive relation to IP-10 (beta coefficient: 33, p=0.02) (Table 3).
Linear regression analysis of factors associated to serum IP-10 levels in acute asthma group.
| Variable | Beta coefficient (95% confidence interval) | p value |
|---|---|---|
| Age | −4.0 (−11.0 to 3.0) | 0.25 |
| Male gender | −49 (−90.7 to 1.4) | 0.06 |
| Using ICS | −7.8 (−34.5 to 18.9) | 0.56 |
| Vitamin D (μg/L) | 1.7 (−2.6 to 6.0) | 0.42 |
| Log cathelicidin (ng/ml) | 33 (4.5 to 62.1) | 0.02 |
| TLR2 (ng/ml) | −6.5 (−28.8 to 15.9) | 0.56 |
| IL-13 (pg/mL) | 2.1 (−3.3 to 7.5) | 0.44 |
| IL-4 (pg/mL) | −18 (−57.0 to 21.5) | 0.36 |
| IL-12 (pg/mL) | −0.1 (−0.3 to 0.1) | 0.43 |
| IFN-γ (IU/mL) | −14 (−122 to 94.1) | 0.79 |
ICS, inhaled corticosteroids.
In the roc analysis, serum IP-10 levels higher than 38.9pg/ml were found to be significantly related to the risk of viral-induced asthma attacks (AUC: 0.701, p=0.002, sensitivity: 85% and specificity: 47%) (Fig. 2b).
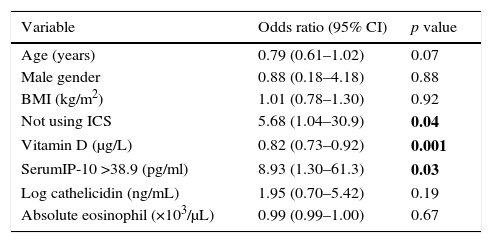
In order to evaluate the relation of IP-10 levels with viral-induced asthma attacks, the risk factors that could relate to acute asthma were included in the multiple analysis of all asthmatics. Serum IP-10 >38.9pg/ml and vit D levels were found to be significantly related to the risk of viral asthma attacks in the logistic regression analysis (IP-10; aOR: 8.93, p=0.03, vit D; aOR: 0.82, p=0.001) independent of age, sex, use of inhaled steroids, BMI, and serum levels of cathelicidin (Table 4).
Multiple logistic regression analysis of possible factors that may affect the development of viral-induced asthma attack.
| Variable | Odds ratio (95% CI) | p value |
|---|---|---|
| Age (years) | 0.79 (0.61–1.02) | 0.07 |
| Male gender | 0.88 (0.18–4.18) | 0.88 |
| BMI (kg/m2) | 1.01 (0.78–1.30) | 0.92 |
| Not using ICS | 5.68 (1.04–30.9) | 0.04 |
| Vitamin D (μg/L) | 0.82 (0.73–0.92) | 0.001 |
| SerumIP-10 >38.9 (pg/ml) | 8.93 (1.30–61.3) | 0.03 |
| Log cathelicidin (ng/mL) | 1.95 (0.70–5.42) | 0.19 |
| Absolute eosinophil (×103/μL) | 0.99 (0.99–1.00) | 0.67 |
ICS, inhaled corticosteroids; BMI, body mass index.
The inflammatory process in asthma, including multiple cell types and mediators, is quite complex.6 Nevertheless, the pathogenesis of viral-induced asthma is clearly different from the pathogenesis of allergen-induced asthma and is poorly understood.3 The present study showed that serum IP-10, and cathelicidin levels were significantly higher and vit D levels were lower in the acute asthma group than the controlled asthma. Serum IP-10 levels showed a significant negative correlation with age. An inverse correlation was found between IP-10, cathelicidin and TLR2 levels. Higher cathelicidin values were found to be positively related to serum IP-10 levels. In the acute asthma group, a significant positive correlation was found between IP-10 and the parameters of asthma attack severity. Moreover, a significant negative correlation was found between serum IP-10 levels and spirometric indices. We demonstrated that high serum IP-10 levels were predictive of viral-induced acute asthma.
Viral respiratory tract infections have long been recognized to be among the most important triggers of asthma attacks.3,20 Most research about the mechanism of viral-induced asthma attacks has focused on rhinovirus.20,21 The identification of impaired innate immunity in asthma suggests a possible mechanism for virus-induced asthma exacerbations.22 Viruses are recognized by TLRs, which are the important components of the innate immune. The interactions between virus and TLR signaling cascades result in the activation of type 1 interferons and pro-inflammatory cytokines and IP-10.21 We also confirmed that serum IP-10 levels were significantly higher in the viral-induced asthma attack group. Another innate immune molecule, human cathelicidin antimicrobial peptide, which is a multifunctional host defence molecule is essential for normal immune responses to infections.23–25 In addition to the antimicrobial effects, cathelicidin also has immunomodulatory properties such as chemoattractant function, inhibition of neutrophil apoptosis, tissue regeneration and cytokine release.26,27 Previous studies suggested that cathelicidin could be used as a marker for a strong systemic immune response in viral or bacterial infections.28,29 Similarly, the current study and our previous report revealed that serum cathelicidin levels were significantly higher in the viral-induced acute asthma group.30
Although the exact reasons for the dependency of IP-10 production on age remain elusive, the reason could be related to Th1-dominant immune responses, such as virus infections. In the study of Kato et al., serum IP-10 showed an inverse correlation with age.31 Similarly, we demonstrated a significant negative correlation between serum IP-10 and age. Further work is needed to explore the relationship between age and IP-10 production.
Type 1 IFNs are an important part of the innate immune response to microbes.32 The antimicrobial peptide, cathelicidin, promotes type 1 IFN production.5 Recently, cathelicidins have been observed to augment interferon-β expression and antiviral activity induced by double-stranded RNA in keratinocytes.33 In the present study, higher cathelicidin values were found to be a significant factor that could influence serum IP-10 levels. We could not measure type 1 IFNs, but higher IP-10 values in the acute asthma group could depend on the overproduction of type 1 IFNs which were possibly upregulated by cathelicidin. We found no significant difference in IFN-γ production between the two asthma groups despite the higher serum IP-10 levels in the acute asthma group. In a recent report, IP-10 was shown to be induced in bronchial epithelial cells infected by rhinovirus through a mechanism that was not dependent on prior induction by either IFN-γ or the type I IFNs.31
Serum cathelicidin levels showed a significant positive correlation with IL-6 and a negative correlation with IL-4 and IL-13 levels in our study. Previous studies showed that human cathelicidin elicited a modest up-regulation of IL-6 gen expression in peripheral blood mononuclear cells.34 The anti-inflammatory properties of cathelicidin are demonstrated by its antagonistic action on IL-4 responses.5 In addition, IP-10 may also contribute to this antagonistic action on IL-4. The overexpression of IP-10 decreased IL-4 release and eosinophil recruitment to the airways in an OVA-sensitized mouse model.35 IP-10 interacts with CXCR3, a receptor that is highly expressed on activated CD4+, CD8+ and NKT cells and stimulates the migration of these cells.11 T cells expressing CXCR3 were associated with Th1 inflammation and IP-10 were even reported to show antagonistic effects on the Th2 cell mediated immune response.36
The modulation of TLR function by cathelicidin can be considered an anti-inflammatory effect.5 Cathelicidin downregulates signaling through TLR4,37 whereas it stabilizes TLR3 ligands and enhances viral responses transmitted via this receptor in bronchial epithelial cells.38 Although the cathelicidin molecule has a general anti-inflammatory effect on TLR stimulation by inhibiting the pro-inflammatory cytokine release from monocytic cells stimulated with TLR2 and TLR4, it can enhance or abrogate inflammatory signals depending on cell type and the microenvironment.4,5 In the present study, significant negative correlations between TLR2 and cathelicidin and IP-10 were shown. A relationship between TLR2 and IP-10 is less well characterized as it has both synergistic and antagonistic effects between TLR2 and TLR3 pathways, which may be stimulus dependent.39,40 Wood et al. showed that IP-10 gen expression in sputum was correlated with TLR2 and TLR3 in viral-induced acute asthma.41
Vit D has several effects on the innate and adaptive immune systems. Vit D has been shown to inhibit IFN-γ gene and protein expression through the Vitamin D receptor-retinoid X receptor complex. Vitamin D receptor agonists can reduce the release of IP-10, a powerful chemokine driving Th1-mediated inflammation.42 Kuo et al. found that 1α,25-(OH) vitamin D could significantly suppress Th1-related chemokine IP-10.43 Moreover, Banerjee et al. found that calcitriol inhibited IP-10 secretion in a concentration-dependent manner in TNFα-treated cells.13 Although we found a negative correlation between vitamin D and IP-10 on the border of significance, lower serum vitamin D levels in viral-induced acute asthma could be considered to contribute to higher IP-10 levels.
The existence of mast cells in the airways is a characteristic feature of asthma.44 The mast cells express the CXCR3 receptor, the only known ligand of which is IP-10. Viral infections may lead to the release of IP-10 in bronchial epithelium that enhances the asthmatic airway inflammation and migration and the activation of mast cells into the airway smooth muscle.45,46 These data clearly indicate that in individuals with pre-existing asthmatic airway inflammation, IP-10 is capable of worsening the inflammation and bronchoconstriction. Moreover, the involvement of IP-10 may explain the lack of corticosteroid efficacy in virus-induced asthma. Wark et al. confirmed that the levels of serum IP-10 of subjects with acute asthma were strongly associated with more severe airflow obstruction and a reduced β2-agonist bronchodilator response.46 A previous study by Medoff et al. showed that IP-10 contributed to airway hyperreactivity and airway inflammation in a mouse model of asthma.8 In the present study, a significant positive correlation was found between IP-10 and the parameters of asthma attack severity in the acute asthma group. Also, a significant negative correlation was found between serum IP-10 levels and spirometric indices.
Finally, the present study demonstrated that serum IP-10 levels were predictive of viral-induced acute asthma. A serum IP-10 value >38.9pg/ml was found to be significantly related (8.9-fold increase) to a viral-induced asthma attack. Similarly, Wark et al. found that increased serum IP-10 levels, in combination with low TNF-α levels, were highly predictive of viral-induced acute asthma. They demonstrated that an increase in IP-10 levels to 168pg/ml or higher was a strong predictor of virus triggered asthma exacerbations.46 Kato et al. also revealed that serum IP-10 was a novel marker of viral-induced acute asthma.31
The limitations of this study are the relatively small sample size and the lack of sputum IP-10, TLRs and cathelicidin levels. Therefore, the correlation between the local immune response in the lungs and IP-10 and cathelicidin levels, which could be more informative, could not be evaluated.
The strengths of this study is that it is the first clinical study that evaluated the roles of IP-10, TLR and cathelicidin simultaneously in viral-induced asthma exacerbations in children. A study demonstrating the association of IP-10 and cathelicidin with viral-induced acute asthma has not been reported in the literature before. In this regard, our study shed light on the pathogenesis of viral-induced acute asthma and also on future studies evaluating IP-10 and cathelicidin as markers of a viral asthma attack. Moreover, this study adds to our understanding of the role of innate and adaptive immunity in viral-induced acute asthma.
In conclusion, our study demonstrated that IP-10 and cathelicidin showed a significant relation to the development of viral asthma exacerbations. These biomarkers can be useful in the clinical management of asthma exacerbations and can also provide potential new treatment targets for acute asthma.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestAll authors declare that there are no conflicts of interest that may be inherent in this submission.