Asthma and/or allergic rhinitis have been associated with sleep disorders. The aim of this study was to evaluate sleep disorders in Latin-American children (4–10 years) from nine countries, with persistent asthma (A) and/or allergic rhinitis (AR) and in normal controls (C).
MethodsParents from 454 C children and 700 A and/or AR children followed up in allergy reference clinics completed the Children's Sleep Habits Questionnaire (CSHQ) which is a retrospective one-week questionnaire composed of 33 questions composed of seven subscales (bedtime resistance, sleep duration, sleep anxiety, night wakings, parasomnias, sleep-disordered breathing and daytime sleepiness). The total scale of CSHQ and the subscales were compared between groups C and A+AR, A (n=285) vs. AR (n=390), and between controlled A (CA, n=103) vs. partially controlled/uncontrolled A (UA, n=182).
ResultsThe comparison between C and A+AR showed no significant differences in age (6.7 years vs. 7.0 years, respectively), mean Body Mass Index and total scale of CSHQ (53.3 vs. 63.2, respectively) and the subscales were significantly higher in the A+AR group. Comparison between groups A and AR, except for sleep anxiety, showed significantly higher values for CSHQ total scale (66.9 vs. 61.0, respectively) and subscales for group A. The UA group showed significantly higher values for total CSHQ scale and subscales in comparison to CA (71.1 vs. 59.4, respectively).
ConclusionsLatin-American children with asthma and/or allergic rhinitis showed sleep disorders identified by the CSHQ when compared to normal controls. Despite being treated, asthma causes sleep impairment, especially when uncontrolled.
Sleep-related disorders (SRD) are associated with sleep behaviour that may be influenced by social, cultural, family, biological and psychological factors. Although the SRD are frequent in children the symptoms are not always noticed and therefore their cause is left untreated.1
It is common for health professionals to fail in specific investigating of these symptoms, however, it is common for parents to omit the SRD symptoms during medical visits, because they do not perceive them as an important issue, either by their ignorance or lack of familiarity with the behaviour deemed appropriate or normal in relation to sleep.2
It is estimated that SRD affect between 25% and 40% of children. Thus, judicious and proper sleep study is important as it allows us to assess whether there are sleep changes or not, as well as whether the child's sleep needs are being met.3
There are several methods (objective and subjective) that allow us to evaluate sleep in children. Although polysomnography (PSG) is considered the best way of assessing SRD, operational and economic constraints make it impossible in the large-scale use, especially in population studies.4
The “sleep diary” is another tool that has been used and should be completed by parents. By collecting only qualitative data, it hinders the overall view of the sleep of several days or weeks, it is inexpensive, time-consuming to complete, and its results are difficult to interpret, as there are no comparison standards.5
Nowadays, written questionnaires (WQ) have been increasingly used due to their good cost-benefit relationship and ease of administration, despite performing subjective and retrospective assessments of children's sleep behaviour.5 There have been several idealised WQ to assess SRD in children of different age groups6–14as well as for the diagnosis of obstructive sleep apnoea.15
One such screening questionnaire designed for use in children is the Children's Sleep Habits Questionnaire (CSHQ) originally available in English.12 It was translated and validated for Portuguese16 and Spanish17 and has proved to be a good tool in the assessment of children with SRD.
Although asthma and allergic rhinitis (AR) are highly prevalent and do interfere with the quality of life of patients, there are few studies evaluating SRD in Latin American children with asthma and/or allergic rhinitis followed up in specialised services.
These children of different populations can be influenced by processes related to their environment (poverty), cultural load (stress, discrimination), the state of their allergic disease (severity, control, tobacco exposure, emergency department visits, poor adherence to treatment) which makes them more vulnerable to increased morbidity and a higher incidence of SRD.18
MethodsChildren (4–10 years) from nine Latin American countries (Argentina, Brazil, Colombia, Cuba, Dominican Republic, Honduras, Mexico, Paraguay, and Uruguay) assisted in specialised allergy services were included in this study. Children with asthma and/or allergic rhinitis and non-allergic controls were enrolled.
Each participating centre was requested to include at least 50 patients and 50 age-matched controls. Children were admitted when they attended for routine visit in each centre participating, from March to April 2014, until the number of settled cases was reached. Patients were classified as having isolated asthma (A)19 or associated with allergic rhinitis (A+AR)20 as well as by the level of asthma control (controlled or partially controlled/uncontrolled asthma).19 Parents and/or guardians signed the inform consent and answered the CSHQ, a retrospective questionnaire that evokes the previous week. In Brazil we used the Portuguese validated version16 and in the other Latin American countries, the Spanish version was used.17 CHSQ questionnaire is constituted by 33 questions and divided into seven subscales: (a) bedtime resistance (goes to bed at same time; falls asleep in own bed; falls asleep in other's bed; needs parent in the room to sleep; struggles at bedtime; afraid of sleeping alone), (b) sleep duration (sleeps too little; sleeps the right amount; sleeps same amount each day), (c) sleep anxiety (needs parent in the room to sleep; afraid of sleeping in the dark; afraid of sleeping alone; trouble sleeping away), (d) night wakings (moves to other bed in the night; awakes once during night; awakes more than once), (e) parasomnias (wets the bed at night; talks during sleep; restless and moves a lot; sleepwalks; grinds teeth during sleep; awakens screaming, sweating; alarmed by scary dream), (f) sleep-disordered breathing (snores loudly; stops breathing; snorts and gaps), and (g) daytime sleepiness (wakes by himself; wakes up in negative mood; others wake child; hard time getting out of bed; takes long time to be alert; seems tired; watching TV; riding in car). Each subscale score was obtained by summing the points given to the questions pertaining thereto and the total scale score was obtained by the sum of the scores of the seven subscales.12
Children's nutritional status was evaluated by Body Mass Index (BMI, weight/height2, Anthro OMS.22 Plus®), compared to World Health Organisation reference and expressed in BMI z score (zBMI).21
CSHQ total scale score and the subscales scores were compared between controls and patients (A+AR) by non-parametric tests (Mann–Whitney). Similar comparisons were made between patients with isolated asthma with those presenting asthma associated with allergic rhinitis, and also regarding to asthma control, i.e., controlled asthma and partially controlled/uncontrolled asthma. Patients with mechanical obstruction of upper airways were excluded from the study.
For all tests it was assumed 5% as the level of rejection of the null hypothesis. All centres had the protocol approved by their Institutional Ethics Committee in accordance with the World Medical Association and the Helsinki Declaration. All parents/guardians signed the informed consent.
ResultsThe control group consisted of 454 children and the group A+AR enrolled 700 patients of which 285 had isolated asthma, 390 asthma associated with allergic rhinitis, and 25 isolated allergic rhinitis. According to asthma control, 103/285 patients were classified as controlled and 182/285 patients were partially controlled/uncontrolled.
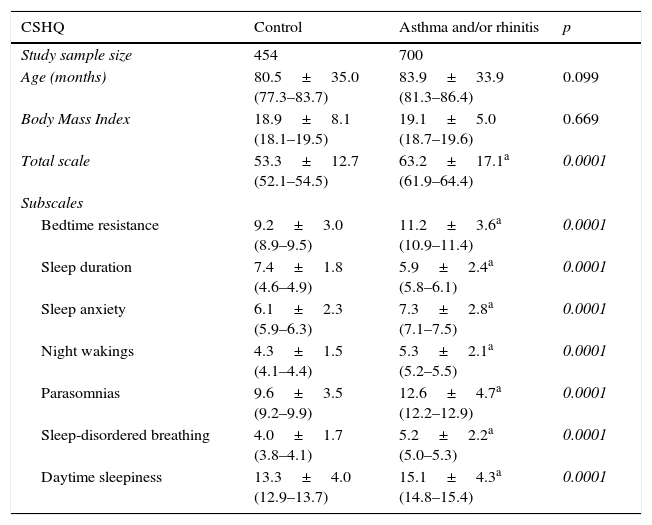
Table 1 summarises the CSHQ total scale score and the subscales scores of controls and patients (A and/or R). We observed that although the two groups were similar in age and BMI, the group of patients had higher scores compared to the control group (except sleep duration, which was higher among controls), suggesting more impaired sleep among patients.
Comparative analysis of Latin American children (controls and with asthma and/or allergic rhinitis) according to age, total scale score and subscale scores of the Children's Sleep Habits Questionnaire (CSHQ).
| CSHQ | Control | Asthma and/or rhinitis | p |
|---|---|---|---|
| Study sample size | 454 | 700 | |
| Age (months) | 80.5±35.0 (77.3–83.7) | 83.9±33.9 (81.3–86.4) | 0.099 |
| Body Mass Index | 18.9±8.1 (18.1–19.5) | 19.1±5.0 (18.7–19.6) | 0.669 |
| Total scale | 53.3±12.7 (52.1–54.5) | 63.2±17.1a (61.9–64.4) | 0.0001 |
| Subscales | |||
| Bedtime resistance | 9.2±3.0 (8.9–9.5) | 11.2±3.6a (10.9–11.4) | 0.0001 |
| Sleep duration | 7.4±1.8 (4.6–4.9) | 5.9±2.4a (5.8–6.1) | 0.0001 |
| Sleep anxiety | 6.1±2.3 (5.9–6.3) | 7.3±2.8a (7.1–7.5) | 0.0001 |
| Night wakings | 4.3±1.5 (4.1–4.4) | 5.3±2.1a (5.2–5.5) | 0.0001 |
| Parasomnias | 9.6±3.5 (9.2–9.9) | 12.6±4.7a (12.2–12.9) | 0.0001 |
| Sleep-disordered breathing | 4.0±1.7 (3.8–4.1) | 5.2±2.2a (5.0–5.3) | 0.0001 |
| Daytime sleepiness | 13.3±4.0 (12.9–13.7) | 15.1±4.3a (14.8–15.4) | 0.0001 |
Mann–Whitney.
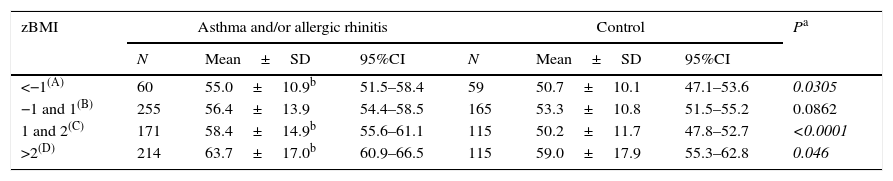
Table 2 shows the CSHQ total scale score of controls and patients according to z score for BMI (zBMI). As we can see there has been an increment in the values of the CSHQ total scale score with increased BMI in both groups. Nevertheless, observing the same zBMI range, the group of patients (A and/or R) had higher values in comparison to controls.
Comparative analysis of total scale score of the Children's Sleep Habits Questionnaire (CSHQ) from Latin American children (controls and with asthma and/or allergic rhinitis) according to Body Mass Index (BMI; z score).
| zBMI | Asthma and/or allergic rhinitis | Control | Pa | ||||
|---|---|---|---|---|---|---|---|
| N | Mean±SD | 95%CI | N | Mean±SD | 95%CI | ||
| <−1(A) | 60 | 55.0±10.9b | 51.5–58.4 | 59 | 50.7±10.1 | 47.1–53.6 | 0.0305 |
| −1 and 1(B) | 255 | 56.4±13.9 | 54.4–58.5 | 165 | 53.3±10.8 | 51.5–55.2 | 0.0862 |
| 1 and 2(C) | 171 | 58.4±14.9b | 55.6–61.1 | 115 | 50.2±11.7 | 47.8–52.7 | <0.0001 |
| >2(D) | 214 | 63.7±17.0b | 60.9–66.5 | 115 | 59.0±17.9 | 55.3–62.8 | 0.046 |
Kruskall–Wallis – asthma and/or allergic rhinitis: p<0.002 – Dunn: B<D.
Control: p<0.002 – Dunn: C<B, D.
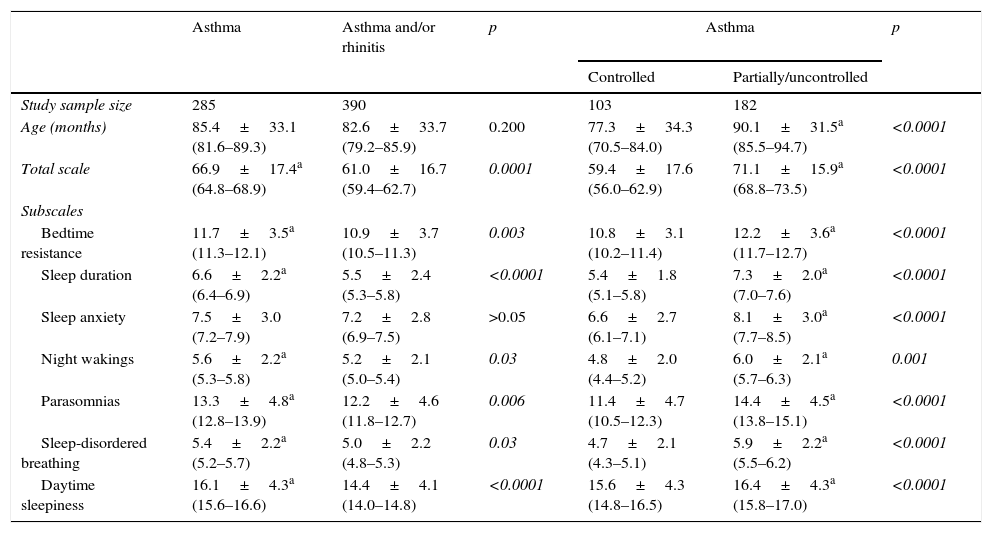
Table 3 presents the data from patients divided according to the presented disease, namely isolated asthma or asthma associated with allergic rhinitis. Except for the sleep anxiety, the group with isolated asthma had greater impairment of sleep than others.
Comparative analysis of Latin American children with isolated or asthma associated with allergic rhinitis, and level of asthma control according to age, total scale score and subscales scores of the Children's Sleep Habits Questionnaire (CSHQ).
| Asthma | Asthma and/or rhinitis | p | Asthma | p | ||
|---|---|---|---|---|---|---|
| Controlled | Partially/uncontrolled | |||||
| Study sample size | 285 | 390 | 103 | 182 | ||
| Age (months) | 85.4±33.1 (81.6–89.3) | 82.6±33.7 (79.2–85.9) | 0.200 | 77.3±34.3 (70.5–84.0) | 90.1±31.5a (85.5–94.7) | <0.0001 |
| Total scale | 66.9±17.4a (64.8–68.9) | 61.0±16.7 (59.4–62.7) | 0.0001 | 59.4±17.6 (56.0–62.9) | 71.1±15.9a (68.8–73.5) | <0.0001 |
| Subscales | ||||||
| Bedtime resistance | 11.7±3.5a (11.3–12.1) | 10.9±3.7 (10.5–11.3) | 0.003 | 10.8±3.1 (10.2–11.4) | 12.2±3.6a (11.7–12.7) | <0.0001 |
| Sleep duration | 6.6±2.2a (6.4–6.9) | 5.5±2.4 (5.3–5.8) | <0.0001 | 5.4±1.8 (5.1–5.8) | 7.3±2.0a (7.0–7.6) | <0.0001 |
| Sleep anxiety | 7.5±3.0 (7.2–7.9) | 7.2±2.8 (6.9–7.5) | >0.05 | 6.6±2.7 (6.1–7.1) | 8.1±3.0a (7.7–8.5) | <0.0001 |
| Night wakings | 5.6±2.2a (5.3–5.8) | 5.2±2.1 (5.0–5.4) | 0.03 | 4.8±2.0 (4.4–5.2) | 6.0±2.1a (5.7–6.3) | 0.001 |
| Parasomnias | 13.3±4.8a (12.8–13.9) | 12.2±4.6 (11.8–12.7) | 0.006 | 11.4±4.7 (10.5–12.3) | 14.4±4.5a (13.8–15.1) | <0.0001 |
| Sleep-disordered breathing | 5.4±2.2a (5.2–5.7) | 5.0±2.2 (4.8–5.3) | 0.03 | 4.7±2.1 (4.3–5.1) | 5.9±2.2a (5.5–6.2) | <0.0001 |
| Daytime sleepiness | 16.1±4.3a (15.6–16.6) | 14.4±4.1 (14.0–14.8) | <0.0001 | 15.6±4.3 (14.8–16.5) | 16.4±4.3a (15.8–17.0) | <0.0001 |
Mann–Whitney.
The comparative analysis of asthmatic patients according to disease control has shown that those with controlled asthma had less sleep disturbances compared to those with partially/uncontrolled (Table 3).
DiscussionThe relationship among atopic diseases, including asthma and allergic rhinitis, and sleep disorders has gained interest, in order to discover the co-existing pathophysiological mechanisms that increase the impairment of sleep and quality of life of these patients.22
High-quality sleep is crucial for the effective development of children in general and in particular, their learning, and the lowering of this ability may indicate that concomitant diseases, including allergy, are poorly controlled or undiagnosed.23,24
The impact of sleep disorders in patients with respiratory allergies has been emphasised by international consensus, such as GINA19 and ARIA (Allergic Rhinitis and its Impact on Asthma)20 which introduced the presence of sleep disorders as a component of the new classification of these diseases.
Studies have shown that children who sleep less at night even though they take naps during the day, have more behavioural problems, and that insufficient and interrupted sleep may be related to serious learning problems, school performance and absenteeism.25–27 A recent meta-analysis has demonstrated clear evidence of poor school performance in children with respiratory sleep disorders.28
In the last years the relationship between allergic diseases and sleep disorders has been widely studied29–31; nevertheless, our study is the first one to analyse this relationship among children from different Latin American countries, having a group of apparently healthy controls from the same localities of allergic patients. This, in a way counteracts the influence of local cultural habits that might interfere with the evaluation of the allergic patients.
The use of CSHQ allowed us to have access to a complete information base on the various sleep disorders in this group of children. As expected, we observed higher values of total CSHQ scale score among children with respiratory allergies when compared to controls, and no differences between the various countries participating in the study (data not shown). This difference occurred on all the subscales constituting the CSHQ (Table 1).
The association between obesity and sleep disorders is well known, the greater the weight the greater the interference with sleep.32 All our children were characterised by zBMI and we observed that the increasing in BMI was associated with increasing total CSHQ scale score for both groups studied. However, when comparing them respecting the same range of zBMI, we continued to observe higher values among those children with A and/or R (Table 2).
The validation of CSHQ either in Portuguese or in Spanish, showed that 41 was the average total score above which the sleep-related problems would be identified.16,17 As we observed, both children groups, controls and patients with A and/or RA, showed an average total CSHQ scale score higher than 41, the cut-off point (Table 1), which was also higher than those observed by other researchers.27–29 It is thought that this difference could be due to the low use of CSHQ in evaluating children with other diseases27 or this cut-off value obtained in validations could be too conservative for the population we studied. Moreover, it is possible that other local factors can act and interfere with the sleep of children a decade after the development of the original study when CSHQ was designed.12
In our study we found a significant association between asthma and sleep disturbances in children with frequent symptoms, results similar to those observed in other studies that also reported a relationship between the severity of asthma and loss of sleep, indicating that the impact on sleep is much more evident when asthma is not controlled or is not diagnosed.23,31,33
Association between increased daytime sleepiness and poorer quality of life, particularly in children with severe AR, has been reported.30 The negative consequences of disturbed sleep caused by uncontrolled AR make children more prone to have excessive daytime sleepiness or chronic non-restful sleep compared to those who rarely or never had symptoms of rhinitis.30
Contrary to the expectations, children with isolated asthma showed higher impairment of sleep when compared with those with asthma associated to allergic rhinitis (Table 3). Although our patients were being monitored in specialised services and receiving treatment, it is possible that this fact has occurred because 64% of those with isolated asthma were partly controlled/uncontrolled. This would generate a higher frequency of nocturnal awakenings and possible impairment of sleep and quality of life of these patients (Table 3).
Often, especially when children are underage, the impaired sleep due to uncontrolled disease extends to family members/caregivers.28,34 It is reported that even when children with respiratory allergies have no symptoms, the sleep of parents/caregivers is compromised by staying awake to control the symptoms of their children, and to have shallow sleep and wake up frequently to make sure that the child is well.35
On the other hand, when they grow older parents/caregivers usually underestimate the sleep problems of these children36 besides being something that is rarely inquired by the attending physician.37
Several authors state that there are still significant gaps in basic knowledge about sleep disorders in children among paediatricians, as well as in the translation of this knowledge into clinical practice and that despite the recognition of the importance of sleep problems, many paediatricians fail to track them properly, especially in older children and adolescents.38
This study has some limitations that might be pointed out: being a cross-sectional study we were unable to establish a cause and effect relationship; sleep quality was assessed by a questionnaire answered by parents/caregivers, with no other objective method (e.g., polysomnography) that could more concretely support our findings.
Nevertheless, we demonstrate, as in other studies,27,28,30 that CSHQ is an accessible and easy to use tool, being sensitive enough to discriminate among different behavioural sleep problems in patients with respiratory allergy from different Latin American countries. While we may question the homogeneity of our sample, constituted by children from different countries and cultures in Latin America, the inclusion of a control group allowed a closer assessment of the reality of each locality.
ConclusionsOur results highlight the possibility that the full charge of the nocturnal symptoms of a child with respiratory allergies and their families/caregivers may not be being recognised by health care providers, on account of lack of knowledge and proper investigation. Thus, research focused on these disorders should be incorporated into any assessment that these patients undergo in order to detect them and allow the incorporation of targeted conducts to reduce its impact on the negative effects of daytime sleepiness, fatigue, behaviour, mood, cognitive functioning and school performance of these children.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe author declare that no patient data appears in this article
Conflict of interestThe authors have no conflict of interest to declare.