INTRODUCTION
Leptin, 16-kDa protein hormone, is produced by white adipose tissue. Structurally, it is a member of the IL-6 family of cytokines. Leptin is an anorexic peptide that is primarily known for its role as a hypothalamic modulator of food intake, body weight and fat stores1.
In addition to its effect on the hypothalamus, leptin is a modulator of the immune and proinflammatory responses. Leptin acts directly on T cells where it enhances the production of Th1 cells promoting inflammation2,3. Leptin also plays a significant role in, e.g., reproductive processes, angiogenesis, hematopoiesis and oxidation of lipids.
On the other hand, leptin had already been shown to be associated with asthma, and there have been reports that asthma and obesity are related4-7. The mechanisms by which leptin may be a risk factor for allergic rhinitis and asthma are not entirely understood, although a few recent studies have addressed this question4-9.
There is a close link between allergic rhinitis and allergic bronchial asthma. The lining of the airway from the nose to the lungs is similar in structure and therefore similarly affected by the allergic process - so what affects the nose also can affect the lungs. And often what happens in one part of the airway has an impact on the other. Although some patients with do not have asthma, inflammatory changes can still be evident in their lower airway. Up to % 30 of allergic rhinitis (AR) patients, with no past history of asthma, will show bronchial hyperreactivity to methacholine10.
A report from the American Academy of Allergy, Asthma, and Immunology estimated that up to 78 % of patients with asthma have nasal symptoms and 38 % of patients with allergic rhinitis have asthma11. Adequate treatment of rhinitis might positively affect the course of asthma, and worsening of rhinitis was associated with persistence of asthma symptoms10.
Because of these relations, various researchers, especially in the last ten years, have referred to allergic rhinitis and asthma as allergic united airway disease10,12-14 and recently as combined AR and asthma syndrome (CARAS)15.
Since, AR and asthma are both mediated by similar allergic inflammatory mechanism, we aimed to evaluate changes of serum leptin concentration and answer the question whether there exists a relationship between leptin levels and allergic rhinitis and asthma or not, and the effect of an inhaler and/or intranasal administered corticosteroid (budesonide) on it.
PATIENTS AND METHODS
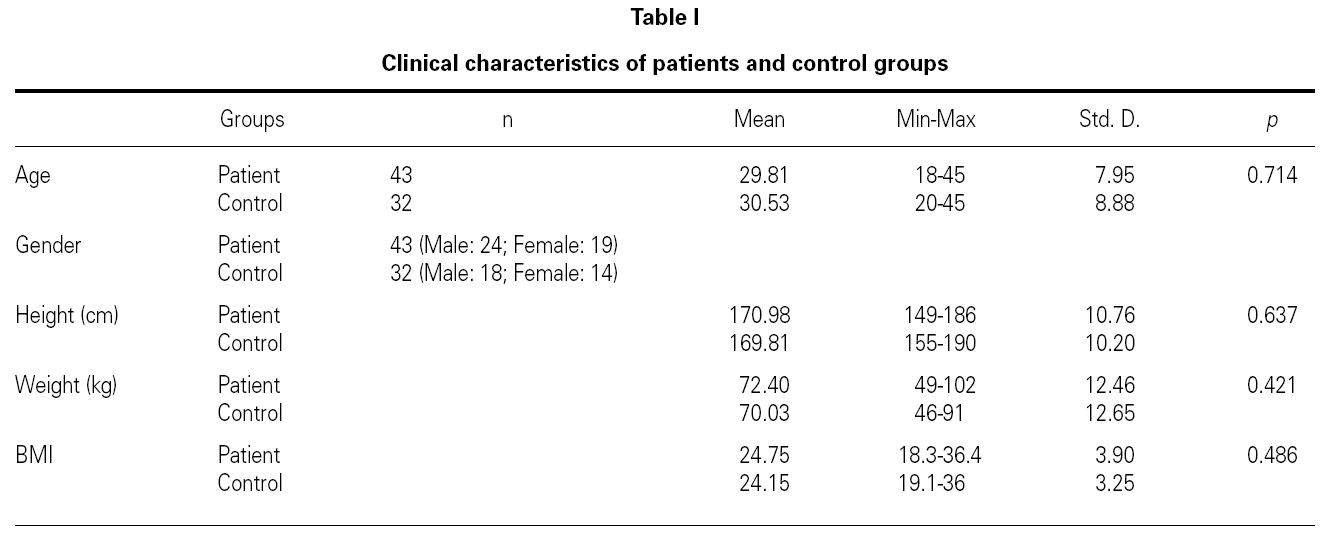
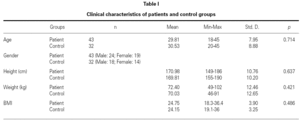
This was a prospective study enrolling 43 patients with allergic rhinitis (10 of with mild asthma) (mean age 29.81, range 18-45 yr) and 32 volunteers as a control group (mean age 30.53, range 20-45 yr). All patients and control groups accepted to participate in the study. Patients were recruited during the symptomatic period in GATA Allergy Clinic, Ankara, Turkey.
Demographic characteristics were recorded and body mass index (BMI) values were calculated (= weight [kg]/height2 (m2)] for all patients (table I). After a 12-hour overnight fast, venous blood samples were collected after 10 to 15 minutes of rest to measure serum leptin, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), eosinophil and total IgE levels. Samples were centrifuged at 4 °C; serum samples were stored at 70 °C until analysis. Low-density lipoprotein-cholesterol (LDL-C) and very low-density lipoprotein-cholesterol (VLDL-C) values were calculated with the Friedewald formula. The principle of the Friedewald formula is based on two assumptions: Firstly, that since very low-density lipoprotein (VLDL) carries most of the circulating triglycerides, VLDL-C can be estimated reasonably well as TG concentrations divided by constant (i.e. 5 for mg/dL and 2.2 for mmol/L). Secondly, that TC is distributed among the three major lipoprotein subclasses [namely HDL, LDL and VLDL. There are though some limitations as to the calculation of LDL-C according to the LDL-F formula, the two most common being that the patient needs to be fasting for at least 12 hours and that TG levels have to be < 400 mg/dL16 Serum leptin levels were measured by a commercially available kit (BIOSOURCE LEPTIN EASIA [BioSource Europe S.A., Nivelles, Belgium]) according to the manufacturer's instructions. (Reference interval in healthy lean adult: lean male 2.4 ± 1.1ng/ml, lean female 6.6 ± 3.0 ng/ml.)
Skin prick tests were performed on the forearms using extracts of grass mix, tree mix, cockroach, mould mix, weed mix, dermatophagoides pteronyssinus, dermatophagoides farinae, dander of dog and cat, negative and positive (histamine) control (Allergo, Stuttgart, Germany). Besides, ultrasonographic examinations performed to evaluate parenchymal changes of liver before and after treatment.
Allergic rhinitis was classified according to the 4 classes of ARIA (mild intermittent, mild persistent, moderate/severe intermittent, moderate/severe persistent), and defined as having a score of 2 or more on a 0 to 3 point scale (0, no symptoms and 3, severe symptoms) regarding to sneezing, itchy nose, running nose, stuffy nose, and eye symptoms17. All patients received nasal budesonide one month (in daily dose of 400 μg).
Asthma was defined and severity was classified according to GINA guidelines and 10 asthmatic patients were additionally treated with a low dose of inhale corticosteroids (400 μg inhale budesonide, daily) and rapid acting β2-agonist, if needed18-20. Asthma symptoms, the number of symptom-free days, use of rescue medication were assessed from asthmatic patients during one month and the rate of asthmatic score was determined. Pulmonary function tests such as forced vital capacity (FVC), forced expiratory volume/1 second (FEV1) and FEV1/FVC were performed to all patients before and after treatment.
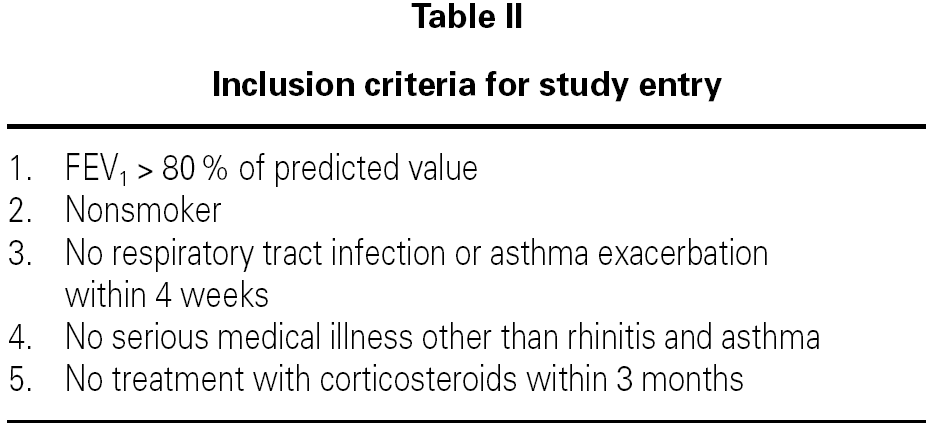
Inclusion Criteria for Study Entry was shown on table II. The University Bioethics Committee approved the study protocol and informed consent was obtained from each patient and volunteer.
Statistical analysis
The statistical analyses were performed using a statistical software package SPSS for windows, SPSS Inc. USA). Independent samples test statistical analysis was used to compare demographic findings of the subjects. In the analyses of presence of asthma, sex and age differences, comparison of serum leptin levels before and after treatment were used Pearson correlation test.
RESULTS
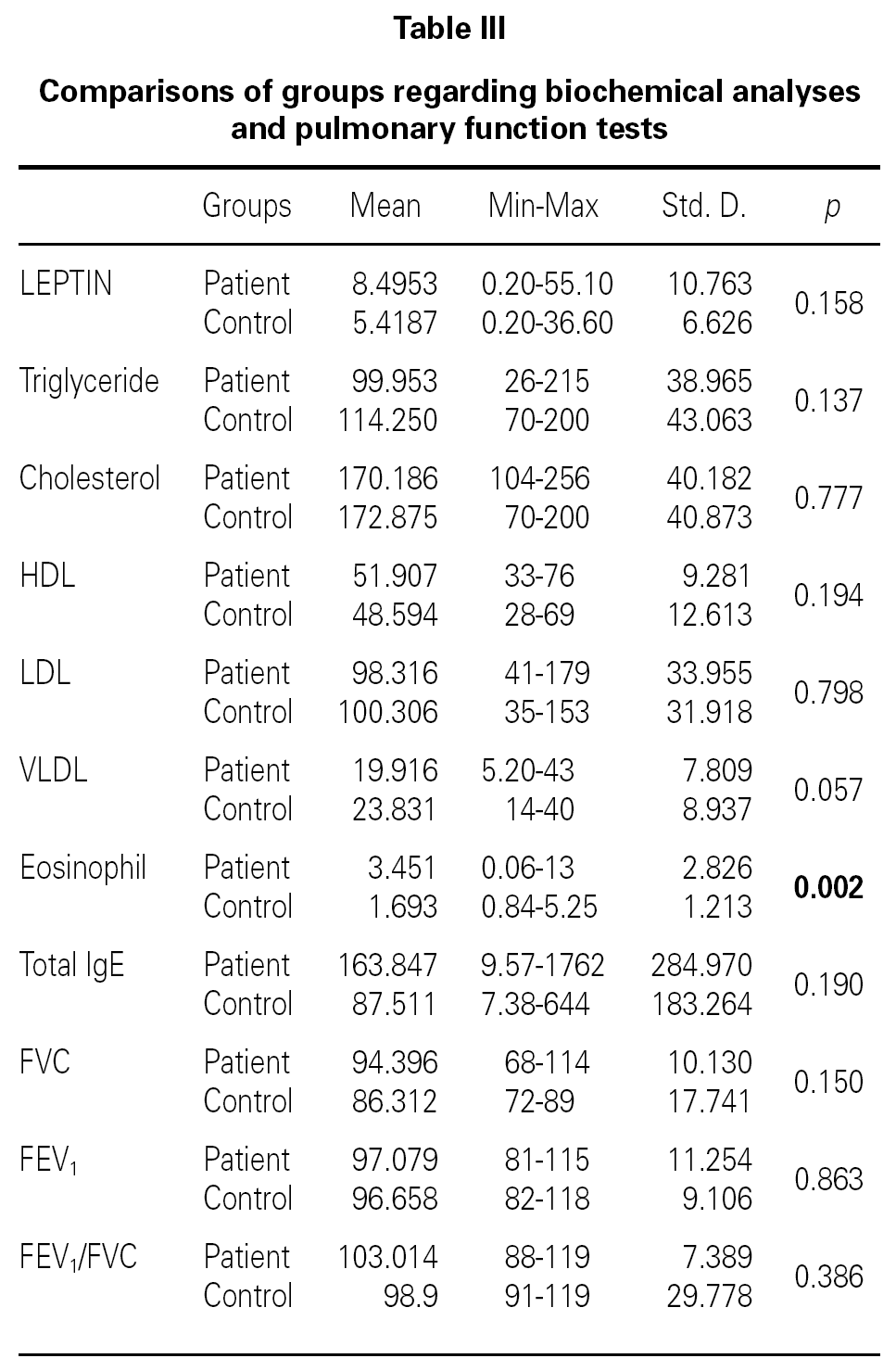
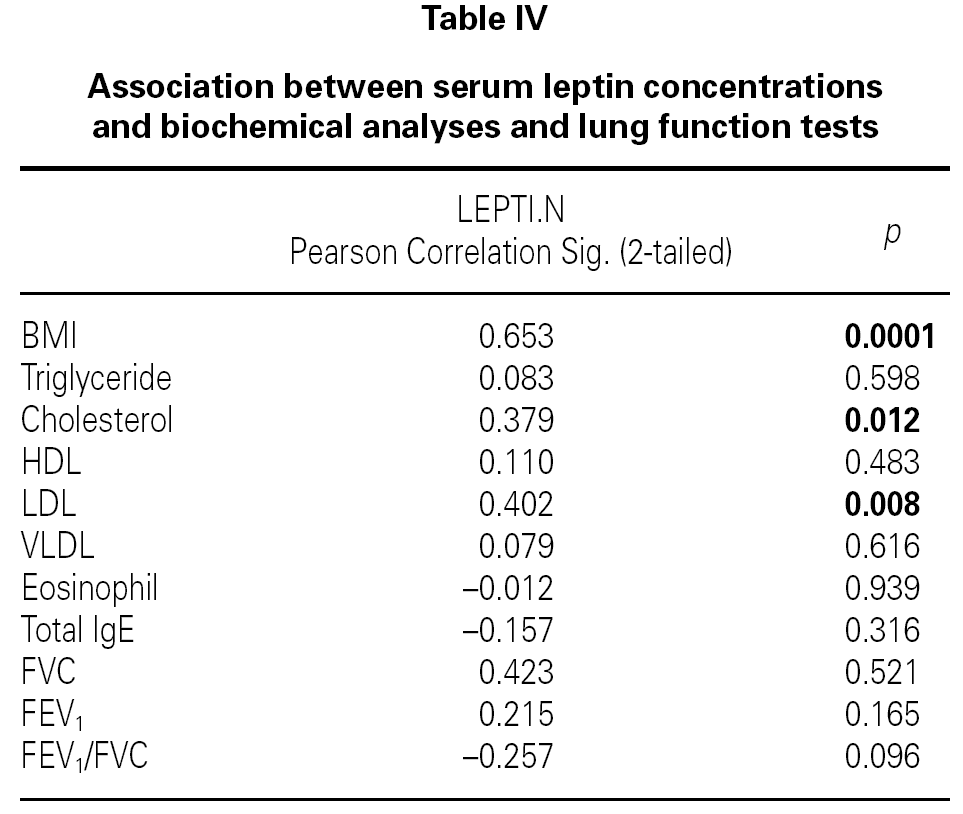
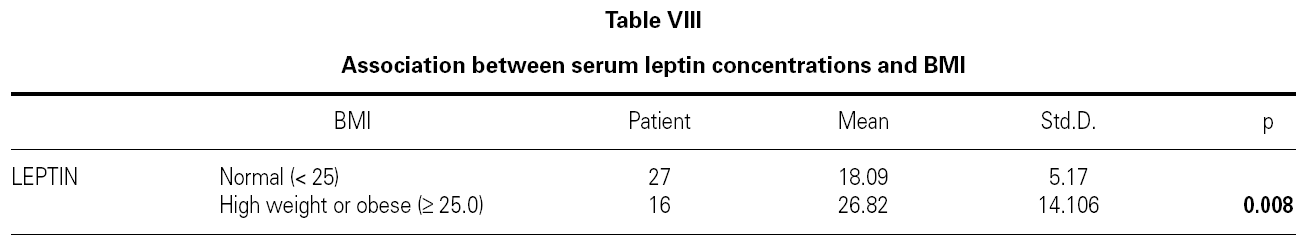
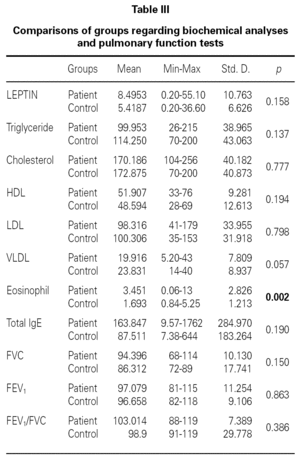
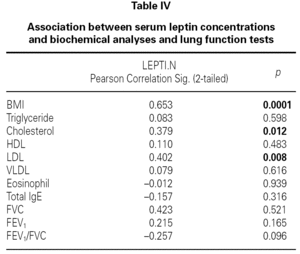
There were no differences between the two groups (patients and volunteers) with respect to demographic characteristics and laboratory analyses (including pulmonary function test), but clear significant differences were noted concerning the eosinophils, an indicator of allergic inflammation (table III). We found a direct link between increased BMI and serum leptin levels (p = 0.0001) (table IV). No association was seen between leptin and triglyceride, HDL-cholesterol, VLDL-cholesterol, eosinophil, total IgE (p > 0.05); except for total cholesterol and LDL-cholesterol (p < 0.05) (table IV).
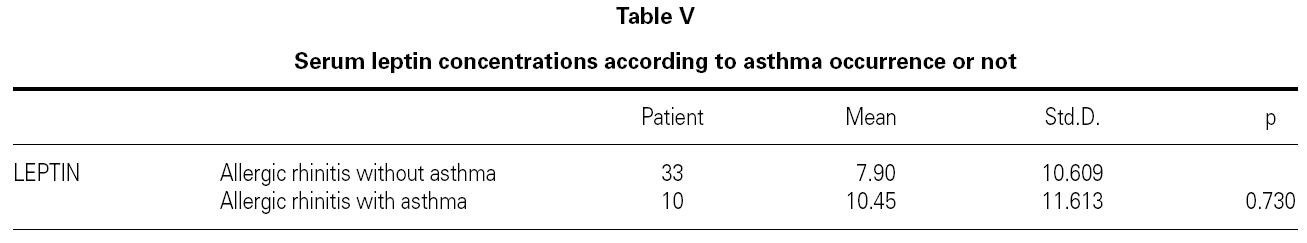
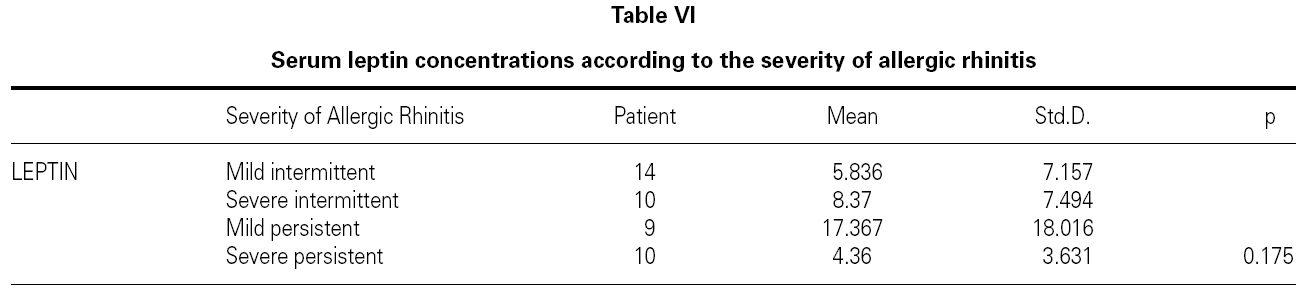
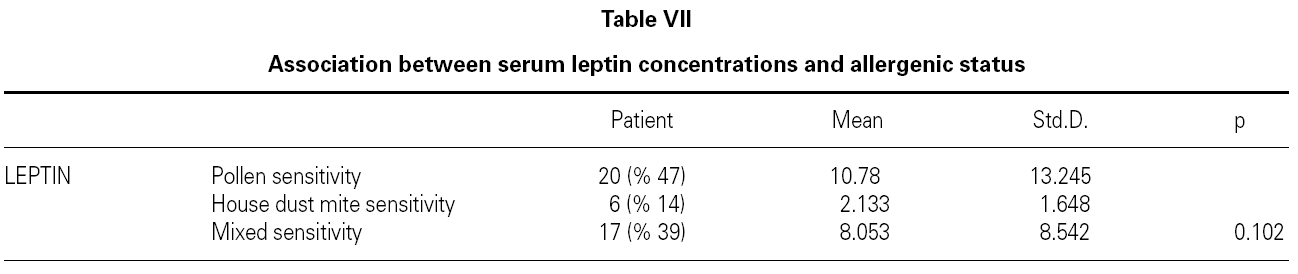
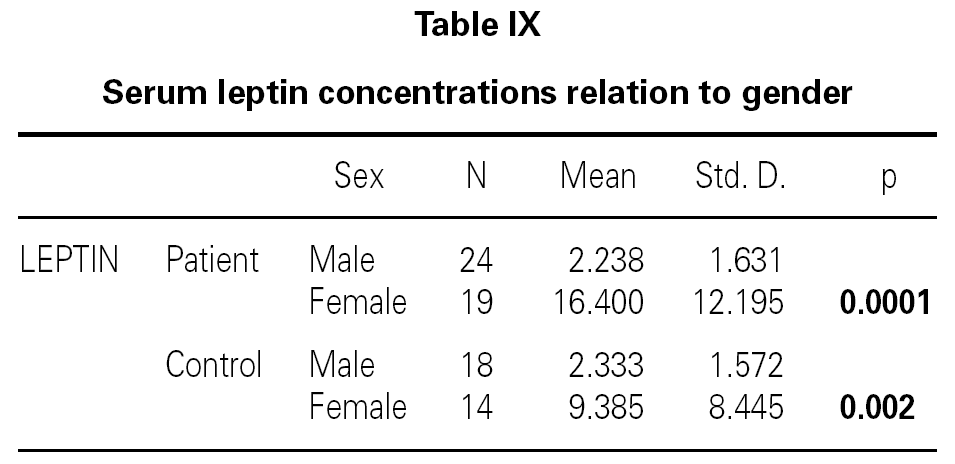
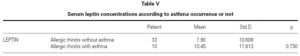
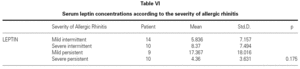
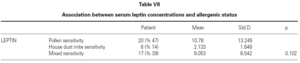
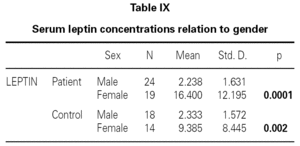
There is no effect to the leptin levels on the severity of allergic rhinitis and asthma existence or not (tables V, VI). Although the allergenic sensitivity seems to be no effect on serum leptin concentrations (p > 0.05) (table VII), leptin level is found higher in pollen sensitivity than house dust mite sensitivity after separating from mix group (p = 0,026). When distributing the patients by bipartite of BMI (as normal and high weight or obese), the leptin levels were found an increase in high weight or obese (p = 0.008) (table VIII). A significant correlation was found between leptin and sex in two groups, in favor of female (p < 0.05) (table IX).
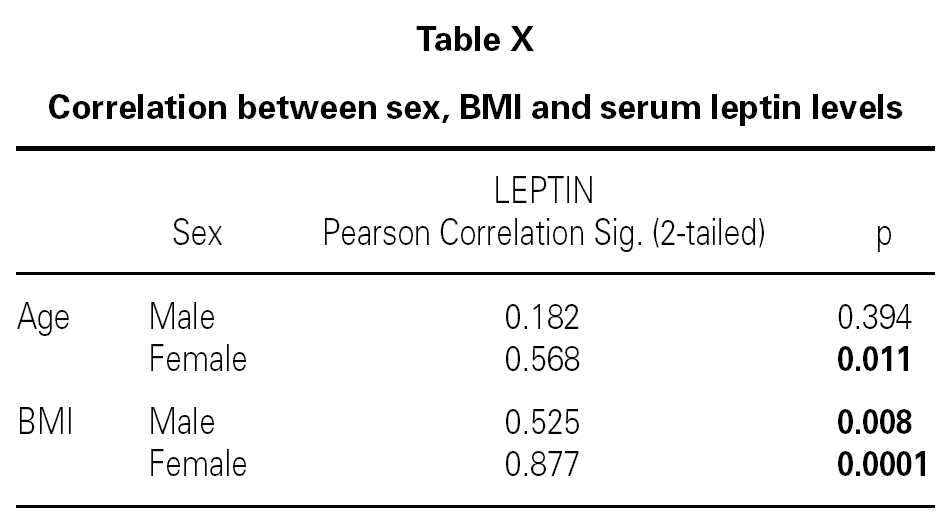
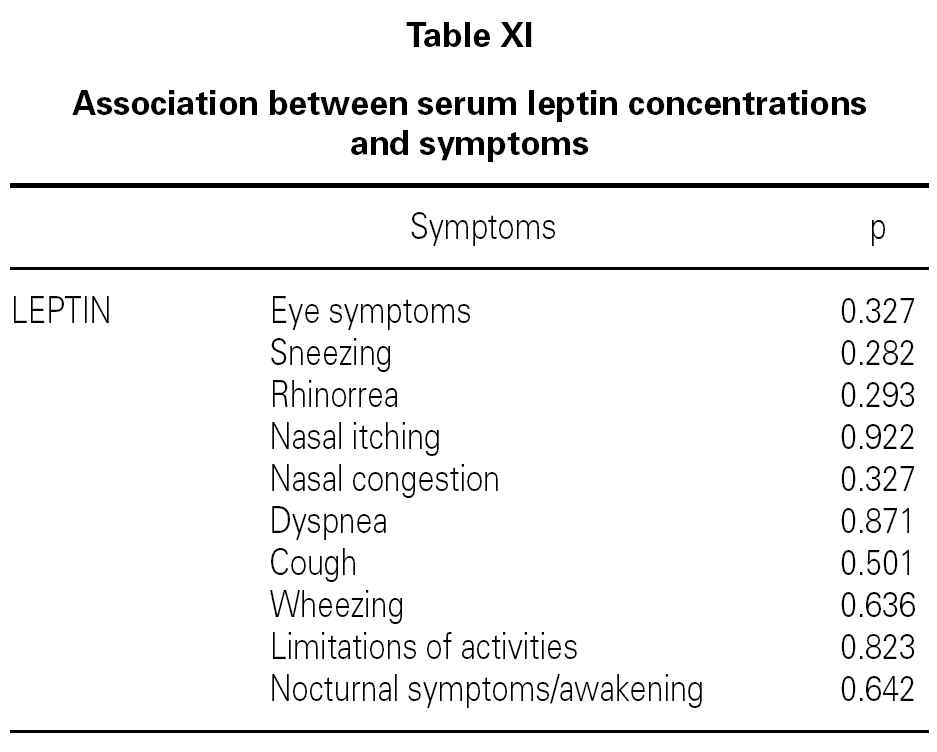
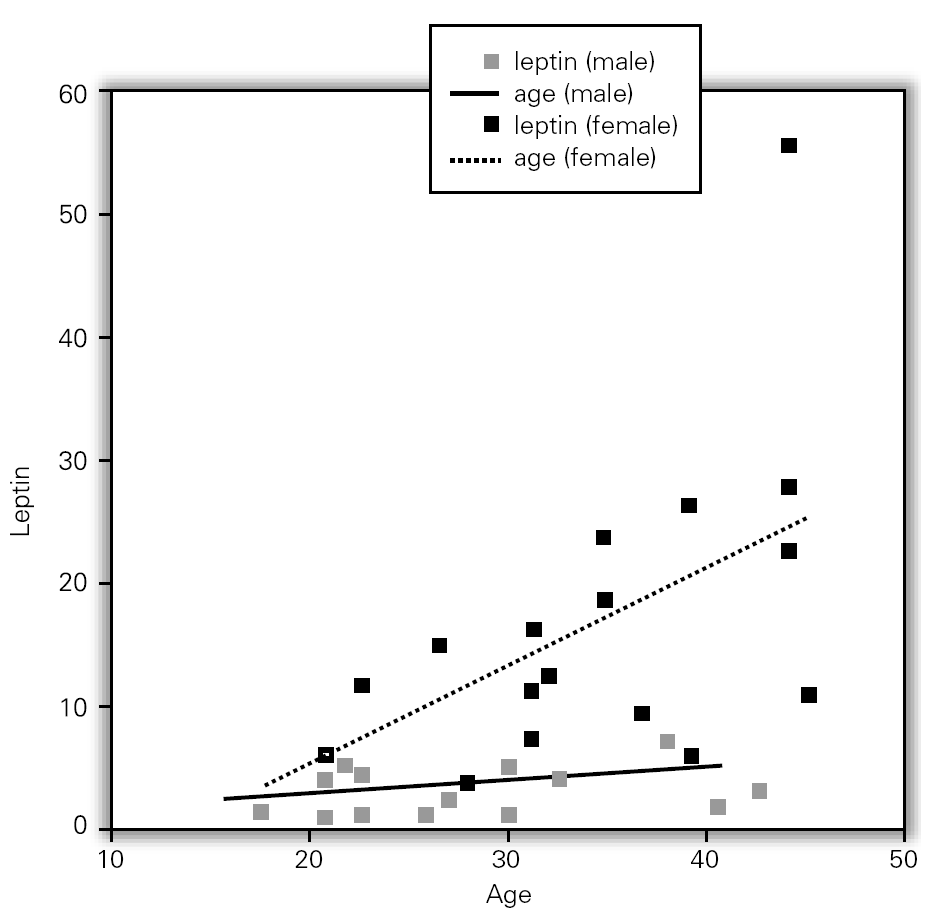
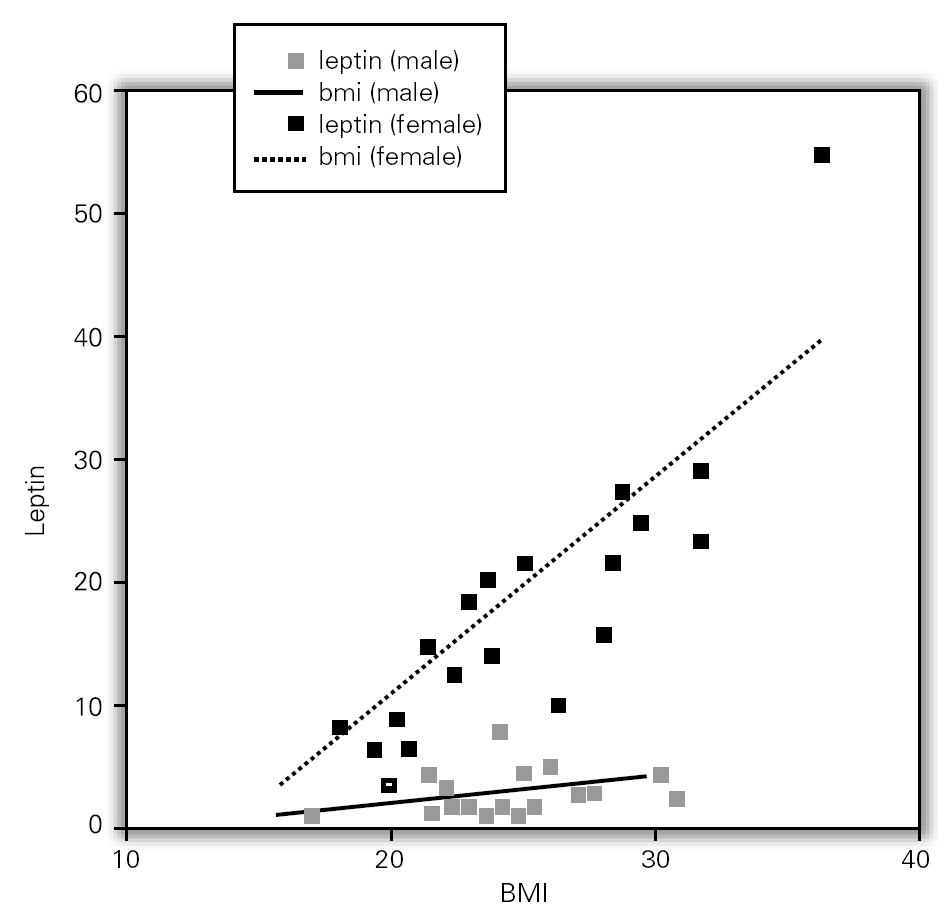
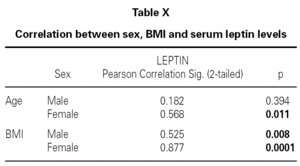
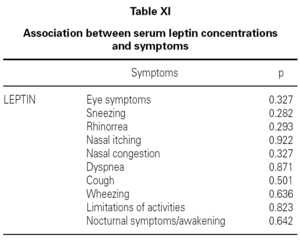
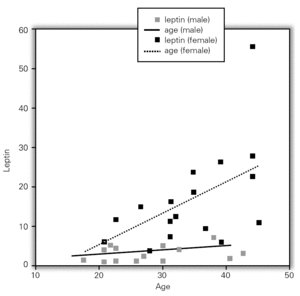
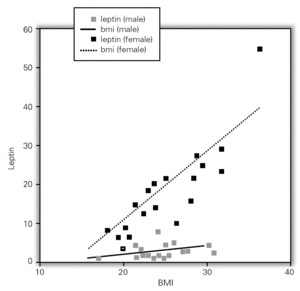
Although, no correlation between allergic rhinitis and mild asthma and serum levels of leptin was shown, these parameters and age correlations were stronger in female than in male, particularly in pre-menopausal women (p = 0.39 for male and p = 0.011 for female), and also found direct link between increased BMI and sex in patient group (p = 0.008 for male and p = 0.0001 for female) (table X). Serum leptin level distribution related to age and BMI were shown in graphic 1 and 2. There was no association between serum leptin level and patient symptoms (table XI).
Figure 1.--Serum leptin level distribution related to age.
Figure 2.--Serum leptin level distribution related to BMI.
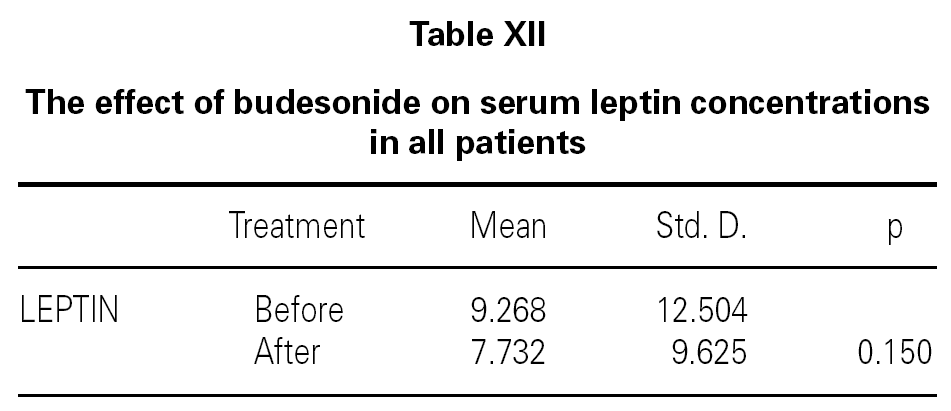
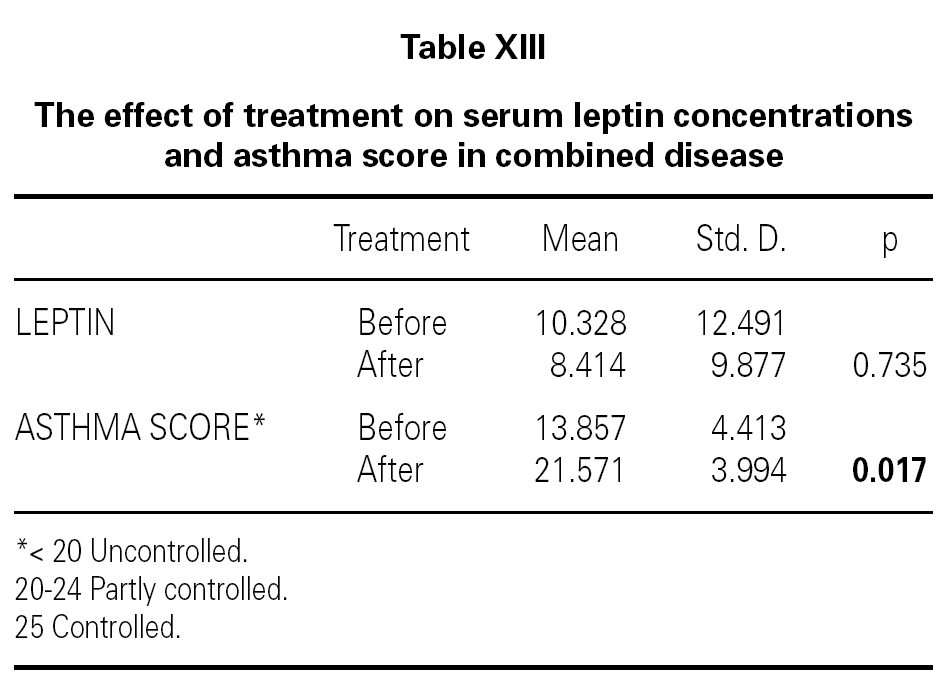
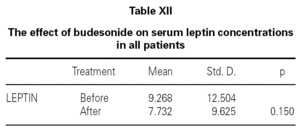
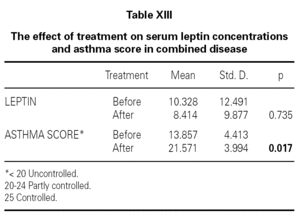
We also determined that there was no effect of intranasal and/or inhaled steroids statistically on serum leptin levels (table XII). It was shown that while asthma score was improving with inhaled and intranasal steroids in allergic rhinitis and mild asthma (p = 0.017), serum leptin level was a little decreased, but not found statistically meaningful (p = 0.735) (table XIII). Lastly, all of ultrasonographic examinations (parenchymal changes of liver) were found normal.
DISCUSSION
Our results indicate that the serum leptin levels in allergic rhinitis and mild asthma are similar to normal groups. Our results also indicate that leptin levels show stronger correlation with BMI, particularly in pre-menopausal women. This result is concordant with literature5,6,21.
We found a significant positive correlation between serum leptin concentrations, cholesterol and LDL. A similar association between leptin levels and cholesterol and triglycerides was observed by Bedir et al. in healthy men22.
Previous studies have demonstrated that, increases in BMI and consequently obesity, have been associated with increased prevelance of asthma. But the mechanisms of this association are not fully understood4,5,23.
Some authors declared that, leptin shares structural and functional homology with IL-6 and it may be directly involved in the regulation of the humoral inflammatory response, stimulating the pro-inflammatory Th1 cytokine pathway and suppressing Th2 cytokine production7,24-29. In other words, leptin influences cytokine production from T lymphocytes, generally switching the phenotype toward a TH1 response30. It's known that leptin stimulates the release of proinflammatory cytokines such as interleukin-6, interferon-g and tumor necrosis factor from the adipose tissue and promotes Th1 immune responses, consequently29.
When we look at the mechanism of allergy, we can see that Th2 cells are responsible for allergic immune responses that they preferentially produce the cytokines IL-4 (that promotes IgE production and inhibits Th1), IL-5 (a growth and differentiation factor for eosinophils) and IL-13 (involved among other pro-allergenic processes in the bronchial tissue re-modulation that occurs in asthma). On the other hand, Th1 cells produce interferon-γ, and IL-2, which inhibit Th2 lymphocytes in experimental models such as in vitro cell cultures30. Actually, a shift from Th2-polarized immune response toward Th1-oriented pattern has been reported after SIT30,31. As a result, leptin may not be involved in allergic pathway, unlike obesity. Our results show the concordant to this situation. On the contrary to our results, some literatures support the hypothesis that leptin plays a key role in allergic process5,8,9.
Systemic administration of exogenous glucocorticoids has been found to increase circulating leptin levels32. But, nasal and/or inhaled budesonide up to 800 micrograms per day does not influence circulating leptin levels. This result is also concordant with literature33.
These results indicate that, the effect of leptin on allergic rhinitis and mild asthma was still unclear and to explain these mechanistic pathways need further large studies.
Correspondence:
Dr. F. Erel
Gülhane Askeri Tp Akademisi
Allerjik Hastalklar BD
06018, Ankara, Türkiye
Fax: + 90-312-304 4139
E-mail: fuatereldr@hotmail.com