The present document offers an update on the recommendations for managing patients with cow's milk allergy – a disorder that manifests in the first year of life, with an estimated prevalence of 1.6–3% in this paediatric age group.
The main causal allergens are the caseins and proteins in lactoserum (beta-lactoglobulin, alpha-lactoalbumin), and the clinical manifestations are highly variable in terms of their presentation and severity. Most allergic reactions affect the skin, followed by the gastrointestinal and respiratory systems, and severe anaphylaxis may occur. The diagnosis of cow's milk allergy is based on the existence of a suggestive clinical history, a positive allergy study and the subsequent application of controlled exposure testing, which constitutes the gold standard for confirming the diagnosis.
The most efficient treatment for cow's milk allergy is an elimination diet and the use of adequate substitution formulas. The elimination diet must include milk from other mammals (e.g., sheep, goat, etc.) due to the risk of cross-reactivity with the proteins of cow's milk.
Most infants with IgE-mediated cow's milk allergy become tolerant in the first few years of life. In those cases where cow's milk allergy persists, novel treatment options may include oral immunotherapy, although most authors do not currently recommend this technique in routine clinical practice.
Enough evidence is not there to confirm the efficacy of elimination diets in the mother and infant for preventing the appearance of cow's milk allergy. Likewise, no benefits have been observed with prebiotic and probiotic dietetic supplements in infants for preventing food allergy.
Cow's milk proteins allergy (CMPA) is an adverse reaction that occurs following the ingestion of cow's milk, and is mediated by an immune mechanism.1 The immune reaction can be IgE-mediated, non-IgE-mediated (cell mediated) or mixed (IgE-mediated and non-IgE mediated). Immunoglobulin E-mediated CMPA is the result of a type I (or immediate) hypersensitivity immune response to one or more milk proteins, and is one of the main forms of allergy, along with allergy to egg, among small children in our setting.2
Cow's milk contains 3g of protein/100ml and includes at least 25 different proteins – all of which may act as antigens.3 The main allergens are the caseins and proteins contained in lactoserum (beta-lactoglobulin, alpha-lactoalbumin, seroalbumin). Immunoglobulin E mediated allergies are characterised by an acute onset (the symptoms usually appear a few minutes after the ingestion of cow's milk, and almost always within 1h) and may comprise skin manifestations (erythema, pruritus, rash and angio-oedema) and/or respiratory alterations (wheezing, stridor, cough, rhinoconjunctivitis) and/or gastrointestinal symptoms (abdominal pain, nausea, vomiting, diarrhoea) or systemic disorders affecting more than one organ (anaphylaxis – a serious and potentially fatal allergic reaction).2
The most efficient treatment for CMPA is a cow's milk protein exclusion diet (elimination diet).2 The use of milk from other mammals (e.g., sheep, goat, etc.) that contain unmodified proteins is not advisable, due to the risk of cross-reactivity with the proteins of cow's milk.4,5 Maternal breastfeeding is the best option, and when breastfeeding is not possible, formulas containing soya proteins, extensively hydrolyzed formulae (eHF) based on cow's milk proteins (CMP), partially hidrolyzed formulae based on rice or amino acids should be started or added. In recent years, specific oral immunotherapy has been developed for CMPA, with the aim of achieving an active immune response. Unless sensitisation accompanied by clinical manifestations are demonstrated, the avoidance of veal consumption is not necessary.2
Epidemiology and PathogenesisAllergy to cow's milk proteins develops early in life and is increasingly frequent in developed countries.5 There is only limited information on the prevalence and clinical characteristics of CMPA. The studies found in the literature differ in terms of patient classification (mixing the concepts IgE-mediated allergy and non-IgE-mediated allergy), with bias referred to patient participation. Furthermore, the lack of universally accepted diagnostic criteria, the rapid evolution of the disease, and the existence of different clinical phenotypes make it difficult to know the true incidence of allergy to cow's milk proteins.6
According to the Alergologica 2005 study, milk and egg are the foods most often implicated in the diagnosis of allergy in patients under 5 years of age, while fruits and nuts are the most frequent causal foods in patients over 5 years of age.7,8
The estimated prevalence of CMPA in the first year of life is 1.6–3%, and decreases to less than 1% in children aged 6 years or older.8,9 Epidemiological data from Israel and Spain (Valencian Community) indicate lower figures, with an incidence of IgE-mediated CMPA of 0.3–0.4% in the first year of life.10,11and pathogenesis
Although a comparative analysis of the protein composition of human and bovine milk reveals certain similarities between them, there are also important differences regarding the types of proteins and their homologies that can cause the immune system to recognise them as foreign.12 Cow's milk contains over 25 different proteins (total 3g of protein/100ml): caseins (alpha(s1)-casein, alpha(s2)-casein, beta-casein and kappa-caseins) and serum (whey) proteins (alpha-lactoalbumin, beta-lactoglobulin, bovine lactoferrin, bovine seroalbumin and bovine immunoglobulins), in proportions of 80% and 20%, respectively. Any of these proteins may act as an allergen, and most patients with IgE-mediated CMPA present polysensitisation.
The predominant milk allergens are caseins, beta-lactoglobulin (no homologous protein being found in human milk) and alpha-lactoalbumin. Sensitisation to bovine seroalbumin may be responsible for the allergy to veal seen in some of these patients. Since the protein is heat sensitive, most studies report good tolerance of cooked veal, with the observation of allergic reactions in 0% (in a study of 234 infants with CMPA of whom 29% were sensitised to veal) to 10% of all patients with CMPA.13–15
The milk of other mammals contains proteins with structures and biological properties similar to those of cow's milk, and therefore can produce cross-reactions. Protein homology is very high with milk from sheep and goats (80–90%), and most patients with CMPA do not tolerate these types of milk. In effect, up to 92% of all patients with CMPA do not tolerate goat's milk. Milk from non-bovid mammals (mare, donkey, camel) shows lesser casein homology and may be tolerated by some patients, although milk of this kind is not readily available.16 According to some publications, mare milk is tolerated by 96%15 and donkey milk is tolerated by 82–96% of all children with CMPA.16,17 Equine milk (mare, donkey) could be an alternative in patients with CMPA, since its composition is similar to that of human milk, and the product is also palatable. However, its production must comply with the necessary specifications referred to hygiene and food safety, and the fat contents must be balanced in order to adapt them to the nutritional requirements of infants and young children.18
Tolerance is the normal immune response to the intake of milk or other foods. The reasons why a small proportion of the population exhibits an abnormal immune response manifesting as hypersensitivity to food components (basically proteins) are not clear. Although hereditary predisposition appears to play a role, the phenotypic expression of allergy seems to be mediated by complex interactions between environmental and genetic factors. The suggested environmental factors include the hygienist, as well as variations in the intake of fatty acids, antioxidants or vitamin D, dual allergenic exposure, and the consumption of processed foods.19,20
The milk antigens come into contact with the immune system fundamentally through the intestine. The intestinal immune system is composed of the luminal barriers (gastric acid and intestinal proteolytic enzymes, IgA, mucus, epithelium), the gut associated lymphoid tissue (GALT: intraepithelial lymphocytes, T lymphocytes of the lamina propria, Peyer's patches, M cells) and the lymphoid organs (mesenteric lymph nodes, spleen, liver). It is in charge of producing tolerogenic responses to non-pathogenic proteins. Such a tolerance is achieved via two primary mechanisms: avoidance of the absorption of intact allergens, and limitation of the deleterious proallergenic immune response to those allergens than gain entry to the system.11 The intestinal immune system is the most frequent sensitisation pathway for food allergens. Other possible sensitisation pathways are the cutaneous and inhalatory routes, which avoid the intestinal tolerance mechanism and thus may facilitate the development of allergic disease.21,22
Different antigen- and host-related factors have been described as being capable of influencing the induction of either food antigen tolerance or sensitisation:
- 1.
Antigen dose: low doses of antigen induce the production of regulatory T cells that promote tolerance via a suppression mechanism. In contrast, anergy or suppression mechanisms intervene in the case of high doses of antigen.23
- 2.
Form of antigen: particles have a stronger sensitising effect than soluble allergens. Food processing also exerts an influence; in this regard, pasteurised milk proteins show increased binding to Peyer's patches, giving rise to an enhanced Th2 response.24
- 3.
Timing of exposure: experimental data suggest that greater tolerance is achieved by introducing important amounts at early ages.
- 4.
Exposure route: extraintestinal food antigen exposure routes are more intensely sensitising.
- 5.
Age: sensitisation to food antigens is more frequent in children – a situation that may be explained by delayed maturation of the intestinal protective mechanisms.
- 6.
Genetic factors: experimental studies have shown genetic susceptibility to play a role in the development of food allergy.
- 7.
Microbiota: the microbial environment of the intestine stimulates the immune system and favours tolerance. Most studies describe an increased risk of food allergy among infants delivered through caesarean section.
- 8.
Exposures that affect the intestinal environment: maternal breastfeeding (favours tolerance), antacid drug treatment (favours sensitisation), Cox-2 inhibitor use (favours loss of tolerance in experimental studies).11
Allergen specific IgE plays a key role in the pathogenesis of IgE-mediated allergy to cow's milk proteins. However, determinations of total serum or allergen-specific IgE for the diagnosis and follow-up of allergy are of variable usefulness. Although many atopic patients present increased total serum IgE titres, such elevations are not specific of allergic disease. There is no cut-off point for distinguishing between patients with allergic disease and the rest of individuals, since important overlapping of the levels is observed.25 Children with very high total serum IgE titres (>10,000kU/l) are at an increased risk of developing severe atopic dermatitis, sensitisation to food and inhalatory allergens, and anaphylaxis, compared with children presenting lower total serum IgE titres (1000–4000kU/l).26
A distinction must be made between sensitisation and allergy. Sensitisation refers to the production of allergen specific IgE, demonstrable by skin testing or in vitro immunoassay for specific IgE. In contrast, allergy is considered when the patient presents allergen-specific IgE and moreover develops symptoms with exposure to the allergen.
When a substance gains entry to the body (through ingestion, inhalation or injection), it is degraded and the allergens (commonly proteins but occasionally also carbohydrates) bind to the antigen-presenting cells (macrophages, dendritic cells, B lymphocytes, etc.). These process the allergen and present complexes composed of peptide fragments together with major histocompatibility (MHC) class II molecules at cell surface level. These surface complexes in turn are recognised by the Th2 cells, which interact with the B lymphocytes, stimulating their maturation to plasmatic cells that in turn produce allergen-specific IgE. Maturation of the B lymphocytes occurs in the mucosal lymphoid tissue. The synthesised allergen-specific IgE is secreted and diffuses through the body, binding to high-affinity receptors (Fc¿RI) of mast cells in tissues and of basophils in blood.
Two steps are therefore required in order for IgE-mediated CMPA to develop. In the first step, sensitisation to cow's milk proteins is established, expressed by the production of specific IgE against cow's milk proteins, which binds to the surface of the mast cells and basophils. In the second step, following exposure to cow's milk proteins, the specific IgE on the cell surface binds on a two-by-two basis to the epitopes of the cow's milk proteins, triggering the release of cell mediators and giving rise to the clinical manifestations of the allergic reaction.1,16
The synthesised allergen-specific IgE binds to amino acid groups of the cow's milk proteins called IgE-binding epitopes. These may be sequential (also called linear) or conformational (amino acids that align when the allergen exhibits its tertiary structure). In food allergy, sequential epitopes appear to be more important, since cooking and digestion would alter the tertiary structure of the allergen, but the linear sequence would be maintained. Some studies have described a correlation between the diversity of sequential IgE-binding epitopes and the persistence of allergy or the severity of the reactions in patients with CMPA.27
A number of studies have compared the epitope recognition profiles of the different milk allergens (alpha(s1)-casein, beta-casein, kappa-casein, alpha-lactoglobulin, beta-lactoglobulin) between patients under 3 years of age with low allergen-specific IgE titres versus older patients with higher allergen-specific IgE titres. The older subjects were found to recognise a greater number of IgE-binding epitopes. In CMPA not only an increased diversity of epitopes is associated to persistent allergy, but also older patients often recognise different sequential epitopes. In addition to the different IgE-binding epitope profiles, it seems that the affinity of these epitopes for allergen-specific IgE plays a role in the pathogenesis of IgE-mediated CMPA.18
Some of the molecular characteristics of the different cow's milk proteins modify their stability, in the same way as certain external factors such as cooking do. The albumins are the predominant proteins in plasma, and bind to different ligands. Their main role is to regulate blood osmotic pressure. Bovine lactoalbumin, although present in small amounts in milk, interacts with phosphatidylcholine (a surfactant present in milk and secreted in the stomach), resulting in slowed rupture of the allergen during digestion. The lipocalins in turn have extracellular ligands with high specificity for hydrophobic molecules. Beta-lactoglobulin, the prevalent whey protein in the milk of ruminants and other mammals, belongs to the lipocalin superfamily, and binding to different molecules (retinol, beta-carotenes, fatty acids, etc.) makes it relatively resistant to acid hydrolysis and degradation by proteases in digestion. In contrast, heat treatment produces conformational changes that increase its susceptibility to enzymatic digestion. In addition, the heat-induced denaturalisation of beta-lactoglobulin is associated to weaker binding to IgE. Cooking lessens whey protein allergenicity, causing some patients with CMPA to tolerate thoroughly cooked dairy products. The processing of milk to yoghourt through fermentation and acidification modifies the whey proteins, allowing patients sensitised only to such proteins to tolerate these foods.28
Clinical manifestationsA number of factors modulate the clinical response in patients with food allergy – some being dependent upon the allergen while others are conditioned to the individual. As a consequence of the interaction of these factors, different clinical–immunological situations can be found, ranging from sensitisation to cow's milk proteins without demonstrable symptoms to the presence of local or generalised manifestations that can affect several organs.
Onset of clinical manifestationsThe onset of symptoms coincides with the introduction of cow's milk in the diet of the patient after a period of breastfeeding. Introduction is usually in the form of infant formulas, but the symptoms can be caused by the introduction of whole milk in paps or directly through fermented dairy products. Since introduction is associated to the end of breastfeeding, in most cases symptoms onset is in the first year of life, and only exceptionally do the manifestations appear after 2 years of age.
Clinical manifestations can appear with first exposure (or apparent first intake) in up to 60% of the cases,13,29 or the patient may tolerate the first exposures. However, the interval between the start of artificial feeding and the appearance of symptoms is usually no longer than one week.
Other less common forms of initial manifestation are through skin contact, directly or indirectly through kisses, caresses, or contact with food consumed by someone else (child or adult).
In very rare cases, and only in the presence of a maternal dietetic overload of cow's milk, symptoms can develop during exclusive maternal breastfeeding as a result of the secretion of bovine proteins in breast milk.30 In these cases the reactions against the cow's milk proteins present in breast milk can occur hours after the intake of cow's milk by the mother.
Development of symptomsThe symptoms usually develop a few minutes after the ingestion of cow's milk, and almost always within 1h after intake. Those reactions that develop several hours or even days after the consumption of cow's milk are usually not mediated by IgE.
Some authors classify food reactions as being of immediate or late onset, or as being of immediate, intermediate or late onset. The presence of IgE type antibodies is observed in the case of immediate reactions, while IgE is usually not found in the other reactions. The latter are encompassed within the concept of non-IgE-mediated allergy. Some very young infants can present immediate type reactions with no evidence of the presence of IgE antibodies at the time of diagnosis.
Clinical manifestationsThe clinical manifestations of immediate type allergic reactions comprise skin symptoms (70–75%), gastrointestinal symptoms (13–34%), respiratory problems (1–8%), alterations affecting more than one organ system (26%), and severe anaphylaxis (1–4%).13
Skin symptoms: Erythema with or without acute urticaria or with an angio-oedematous component is found in a little over 50% of all patients. Swelling of the eyelids, lips and/or hands and feet may be observed. Between 10 and 15% of the total patient population experience only local symptoms such as perioral erythema after ingestion of the food. These mild presentations may precede other episodes of greater intensity. Some patients develop symptoms before the introduction of artificial feeding, with erythema or urticaria in areas that have come into accidental contact with milk.
Allergy to cow's milk proteins is often associated to manifestations of atopic dermatitis – the latter being a very common problem in the first months of life that manifests as outbreaks which may seem to worsen or coincide with the ingestion of cow's milk proteins. This often leads to confusion as to the true origin of the clinical Picture.31,32 In such cases, if dermatitis is the only clinical manifestation, an elimination diet should be provided for 2–3 weeks, followed by controlled provocation testing to confirm cow's milk as the cause of the problem, before excluding this food from the diet.33
Gastrointestinal symptoms: Patients may suffer immediate vomiting of a non-specific nature, i.e., indistinguishable from vomiting due to other causes. In infants under 12 months of age there have been descriptions of associations between CMPA and gastro-oesophageal reflux and diarrhoea.34,35
Systematic rejection of the bottle, together with crying and irritability, in nursing infants without other manifestations of disease may be early signs suggestive of CMPA, although such symptoms are soon followed by other more objective manifestations.
Respiratory symptoms: Acute rhinoconjunctivitis with watery nasal secretion, sneezing and tearing are often seen in controlled provocation tests36 and are also observed among the initial clinical manifestations, but are less often reported by the parents, since they precede other more alarming symptoms. Self-limited mild dysphonic episodes may be observed.
Lower airway breathing difficulty due to bronchospasm or conditions suggestive of glottic oedema with breathing difficulty and inspiratory stridor can be observed, in all cases on an acute basis immediately after ingestion of the causal food. These symptoms do not usually appear in isolation but are accompanied by other systemic manifestations, and can give rise to immediate life-threatening situations.
Anaphylaxis: Clinical situations of anaphylaxis can be classified as severe bronchospasm, epiglottic oedema or life-threatening anaphylactic shock, and as less serious generalised conditions involving more than one organ.
The clinical manifestations of anaphylaxis can appear with the first food exposures in the nursing infant, and the risk increases with age and the persistence of sensitisation.
There are no data on the incidence and prevalence of anaphylaxis due to cow's milk proteins.37 The incidence of anaphylaxis is three-fold greater in infants in the first 4 years of life than in the general population, and cow's milk is one of the main implicated foods.38 Serious clinical manifestations are frequent in patients over 4–5 years of age and with persistent CMPA.39 Serious symptoms posing a threat to the life of the patient have been related to sudden death by some authors.40
Diagnosis of allergy to cow's milk proteinsThe diagnosis of IgE-mediated cow's milk allergy should be based on suggestive clinical manifestations as described above, and is confirmed or discarded by the findings of a full allergic study including skin prick tests and/or in vitro tests with the allergenic fractions of milk. Controlled exposure testing is often needed.41
This methodological approach should be rigorous, since consultations due to adverse reactions with milk referred to specialised centres as possible cases of CMPA are ultimately confirmed as IgE-mediated allergies in only about 30–60% of the cases.13,42,43
In infancy the diagnosis of CMPA should be regularly revised and updated, since the clinical manifestations are transient in most cases. Maintaining a diagnosis of CMPA, with the consequent observation of an elimination diet in a patient who might actually be tolerant, generates personal, family and healthcare services costs that should be avoided.
The sequence/frequency with which diagnostic revision should be carried out after the first diagnosis has not been well established, and is one of the factors conditioning the important variability in current allergy prevalence rates reported in the literature.44–46
Clinical historyInitial diagnosisSeverity. The severity of the condition is dependent upon the degree of sensitisation and the amount ingested. In some scantly sensitised patients it may be necessary to reach a high threshold dose in order to produce symptoms, while anaphylactic patients may suffer serious problems with only small amounts of milk protein.
Evolution of symptoms with changes in diet. Disappearance of the symptoms after temporarily suppressing cow's milk proteins and replacing them with maternal breastfeeding or special formulas is suggestive of a positive diagnosis of CMPA, while persistence of the symptoms discards such a diagnosis.
Physical examinationA full patient exploration is required, placing special emphasis on the following aspects:
Evaluation of the progression of body weight and height.
Skin examination, documenting symptoms of atopic dermatitis in all parts of the body, with special attention to perioral lesions that may worsen on coming into contact with milk, and dermographism.
The exploration sequence is similar in older children. The progression of body weight and height should be assessed, particularly in patients polysensitised to several foods.
Detection of milk and milk protein specific IgE antibodiesSkin testsSkin testing is performed via the intraepidermal route using the prick technique with whole milk or the milk protein fractions corresponding to beta-lactoglobulin (BLG), alpha-lactoalbumin (ALA) and casein completed with bovine seroalbumin (BSA) when allergy to veal is suspected, adopting a standardised technique.47 A positive reading is represented by a papule (wheal) measuring 3mm or more in size. There is no age limitation for performing this technique.
The sensitivity of skin testing with milk is highly variable (50–100%),48,49 depending on the age of the patient (more erythema being observed in small infants) and possibly also on the extract used.
The concentrations of the extracts currently available in Spain vary among the different commercial sources from 5–20mg/ml for whey proteins, casein and whole milk. This broad variability makes it difficult to interpret the tests, and in this sense optimisation and homogenisation of the extracts is an essential requirement.
Some authors use whole milk from the commercial container as antigen in prick testing. This source contains approximately 30mg/ml of proteins and affords greater sensitivity. However, some authors consider positivity to several milk fractions to be more sensitive.4
The conduction of protein fraction sensitisation studies in both skin tests and in vitro is useful for seeking increased sensitivity (initially beta-lactoglobulin) and prognostic performance (casein). The sensitivity and specificity of the tests must be established for each population group, and are not always extrapolatable to other populations or age categories.
In our setting, some authors have recorded a sensitivity of 99% and a specificity of 38%, with a negative predictive value (NPV) of 97% and a positive predictive value (PPV) of 56%, among infants under 1 year of age and using commercial antigens.50
Likewise, using commercial antigens and in patients with atopic dermatitis and covering a broad age range, some investigators have obtained a sensitivity of 94% and a specificity of 46%.51
Attempts have been made to relate papule (wheal) size to tolerance status, and in this regard some work groups have established different cut-off points according to patient age,52 such as 6mm in infants under 2 years of age and 8mm in those over 2 years of age, affording specificities of 100% but low sensitivity values.
A negative predictive value of 98% has been recorded with milk for consumption.4
In sum, a negative prick test is a good way to rule out sensitisation to milk, while a positive prick test has a lesser discriminating capacity.
Intradermal tests might cause systemic reactions and therefore should not be used.53 On the other hand, while prick tests are safe, there have been exceptional reports of systemic reactions.54
The patch tests with commercial antigens used by some authors are not recommended in IgE-mediated allergy.24
For establishing an aetiological diagnosis in patients with atopic dermatitis, some authors find it useful to perform epicutaneous tests with powdered milk,55–57 adopting a conventional technique. However, according to other investigators, such tests are very non-specific, irritating and scantly discriminating.58
Determination of serum specific IgESerum specific IgE allows objective and quantitative assessment of sensitisation to an allergen. In this case determination can be made for whole milk and/or milk fractions.
Negative specific IgE testing for whole milk always implies negative testing for its different protein fractions, and so performing milk fraction tests is therefore not necessary. In clinical practice a value of 0.35kUA/l is used as positivity cut-off point. Determinations can be made in application to cow's milk, α-lactoalbumin (Bos d4), β-lactoglobulin (Bos d5), bovine seroalbumin (Bos d6), casein (Bos d8), and goat's milk.
If specific IgE for milk proves positive, separate determination in relation to its different protein fractions is of prognostic value, since in the course of follow-up of the process the observation of decreasing beta-lactoglobulin and casein values has been related to tolerance.59 It is not clear whether the magnitude of the IgE titres is associated to the severity of the symptoms. However, it does seem clear that initially high or low values do not allow us to identify in whom or when tolerance will develop60,61 – although a drop in values is effectively associated to an increased likelihood of tolerance.62
Different IgE cut-off values orienting towards tolerance have been proposed. In our setting, and considering only situations during the first year of life, an ImmunoCAP (CAP) test value for milk of 2.5 kUA/l or more would offer strong positive predictive value (PPV: 90%) for a lack of tolerance.21 In relation to older patient ages, a recent Spanish publication on the diagnosis and evolution of CMPA carried out in 13 centres and in 327 patients found specific IgE titres for casein of 1.22kU/l (18 months), 3kU/l (24 months), 2.39kU/l (36 months) and 2.73kU/l (48 months) to predict clinical reactivity to cow's milk with a PPV of 90%.63
The values vary according to the true prevalence of the disease (established by provocation testing) in the patients studied in each case. With these limitations (all the figures are variable depending on the prevalence of the disease in the studied population), and in a very generic and global manner, the probability of presenting clinical manifestations with the ingestion of milk is greater the higher the specific IgE titre for milk and casein, and the larger the skin test diameter readings.
There are other lines of research that explore factors which allow us to predict tolerance or the persistence of allergy. Studies in this field include analysis of the recognition of certain linear epitopes of beta-lactoglobulin and casein by the serum of patients with persistent allergy to cow's milk64–67 or the secretion of TNF-alpha, which could allow us to predict tolerance and distinguish between skin symptoms and gastrointestinal manifestations.68 Such applications have however not yet received applications in daily clinical practice.
Other determinations such as IgG and its components or microarray techniques offer no advantages.69 Likewise, basophil activation testing has not yielded data allowing us to improve upon the results of the existing diagnostic techniques.70,71
A promising and very recently developed approach that remains to be validated in different populations involves a normogram with six variables: skin test measurement, specific IgE titre, total minus specific IgE, symptoms, sex and age.72
In preliminary studies, the mentioned normogram has allowed calculation of the probability of a clinical reaction to cow's milk with a sensitivity of 93% and a specificity of 89%. This model, designed in the Irish population and experimentally tested in the Canadian population,73 is pending further validations that may allow it to be applied to decision making, and may prove very useful.
To summarise, in addition to the mentioned data, which vary considerably according to the author and population involved (although they can provide an orientation for deciding both the time and nature of controlled exposure testing), the clinician must take into account the time elapsed since the last episode, its severity, and the presence or absence of recent clinical manifestations in response to minimal or accidental contacts.
Controlled exposure testingThe only definitive method for demonstrating tolerance or non-tolerance is the controlled exposure test – more widely known as the provocation test.
This test can pose risks for the patient, is time consuming for both the patient and relatives, and involves considerable costs in terms of time and resources for the healthcare system. It is therefore advisable to limit testing to those cases in which the benefits compensate the costs involved.
Although the gold standard is always double-blind testing with placebo, use of open provocation testing or simple-blind testing (in certain cases with doubtful symptoms) is accepted in daily clinical practice – double-blind testing being reserved for research work.
In any case, a positive skin test or specific IgE result for milk, with a recent clinical episode (within the last 3 months) in the first year of life, make it unnecessary to perform a diagnostic exposure test.
However, before starting treatment based on immunotherapy for milk and/or biological treatments, and with the purpose of determining the clinical reactivity threshold, a careful controlled exposure test may prove necessary even in cases with recent clinical manifestations.
Controlled milk exposure testing techniquePrior requirements- 1.
Before testing, the relatives of the patient must receive a detailed explanation describing the purpose of the test and the details of the procedure. Patient acceptance is to be reflected by a signed consent document, which is to be filed with the patient history. The relatives are to receive a copy of this document.
- 2.
The patient must be accompanied at all times by a relative or caregiver authorised by the person signing the consent document.
- 3.
The patient must be asymptomatic or at least stable before the test, with adequate control of any possible disorders.
- 4.
In principle, medication of any kind is to be avoided, except as regards those treatments essential for controlling chronic symptoms (atopic dermatitis, asthma etc.). The suspension of any medication is to be made at least a few days before testing in order to prevent prior symptoms rebound from interfering with the results.
- 5.
The patient is to avoid food intake for at least 3h before testing.
- 6.
The personnel performing the test must be adequately trained to deal with potentially serious anaphylactic reactions, and the necessary medication must be available.
- 7.
The administered product or products (active drugs and placebos) must be duly identified with the name of the patient in order to avoid any possible confusion.
- 1.
An infant formula at the usual concentration should always be used during the first months of life. In infants over 1 year of age, the provocation test can be performed with whole cow's milk.
- 2.
The patient is to remain under observation for at least 1–2h after the last intake.
- 3.
The administration regimen varies according to the clinical conditions and the results of the allergy study. In practice, the minimum dose for developing symptoms is at least 1ml, although lesser doses can be used depending on the previous symptoms or if excessive concern on the part of the patient or relatives might interfere with the results. In these cases simple or even double-blind testing is indicated.
- 4.
A recommended administration regimen used in clinical practice in our setting3 involves testing in a single day with successive doses of 2ml, 5ml, 10ml, 25ml, 50ml and 120ml, spaced at least 30min apart, until the amount corresponding to the age of the child is reached. In some cases, for time or operative reasons, testing may have to be prolonged over a two-day period. In any case, the amounts involved can be varied according to clinician criterion, with due assessment of the potential risks.
The test will be considered positive and is to be stopped if any of the symptoms defined as being diagnostic of allergy develop during the test or in the subsequent 60–120min of observation once the test is over.
If symptoms appear, the patient must receive treatment on an early basis and until complete remission is achieved. Once the symptoms have been controlled, the patient is to remain under observation for at least another 60–120min. Information is to be provided on the signs to be noted and on the measures to be taken in the home. In some cases the continuation of drug treatment may be necessary in the hours following the test. When provocation testing proves positive, a strict elimination diet without CMP is to be prescribed.
Patients extremely sensitive to CMP may present positive skin tests with casein hydrolysates. In these cases controlled exposure testing with the hydrolysate is required in order to check tolerance before it is incorporated to the diet. This measure is not necessary with products from other sources (rice, soya) or elemental amino acid formulas.
Negative testingIf tolerance is confirmed, administration of the formula or whole milk in the home should be continued on the same day or on the next day, without free intervals in between.
A few patients tolerate milk in controlled provocation testing and for a subsequent period of 4–5 days only to develop the symptoms again later on. Tolerance is not considered to have been reached until it is confirmed that the patient is able to consume milk in normal amounts for his or her age during one week.74,75
Elimination diet and recording of symptomsIn patients with chronic symptoms such as atopic dermatitis and urticaria, if the allergy study proves positive, a strict elimination diet without milk or products containing milk is to be prescribed. In breastfeeding infants, the mother is to receive diet instructions. The diet restriction period should be short in all cases (no more than 2–3 weeks), and is to be accompanied by a symptoms registry.
The diet is considered to have been effective if the symptoms disappear or if significant clinical improvement is noted. If no improvement is observed, milk should be incorporated into the diet again. However, if the patient is seen to improve, diagnostic provocation testing is indicated, and if the test proves positive, with acute clinical manifestations or dermatitis, a firm diagnosis of CMPA can be considered. In contrast, if provocation testing proves negative, the food should be reincorporated into the diet and the patient is to be kept under observation for a few days.
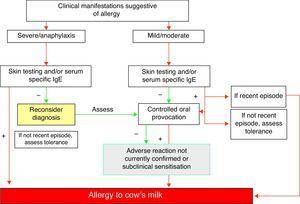
Diagnostic summary of IgE-mediated cow's milk allergy (Fig. 1)- 1.
A compatible history with characteristic immediate type allergic symptoms.
- 2.
Evidence of specific IgE as established from skin tests and/or serum specific IgE for milk.
- 3.
Recent evident clinical manifestations or positive controlled exposure testing.
- 4.
The diagnosis must be re-evaluated on a periodic basis.
The treatment of CMPA is based on the introduction of an elimination diet, with the following aims in mind:
- 1.
Complete disappearance of the clinical symptoms.
- 2.
Adequate patient feeding, guaranteeing all the nutrients needed for normal growth and development in this critical period of life. This aspect is particularly important in patients with multiple food allergies.76
The treatment of CMPA requires a correct diagnosis, since an elimination diet that is not really indicated can alter patient growth and have a negative impact upon the quality of life of the child and family, with the generation of unnecessary economical costs.
Transgressions are relatively frequent in this context, and can give rise to serious reactions manifesting in routine situations of daily life.77
Food allergy, including CMPA, is the allergic disease that generates most economical costs, totalling over 4000 Euros per patient, of which substitution formulas account for almost 800 Euros during the first year of life and over 1100 Euros during the second year.78 Accordingly, once we have been able to control the symptoms of CMPA, our next aim must be to secure tolerance as quickly as possible with a view to restoring a normal diet, improving quality of life and lessening the economical costs.
A number of situations can be found in children with CMPA, depending on the age of the patient and the type of feeding received:
Infants with CMPA receiving exclusive maternal breastfeeding- 1.
Cow's milk proteins have been found to be present in breast milk,79,80 although cases involving clinical manifestations due to IgE-mediated allergy in infants receiving exclusive maternal breastfeeding are exceptional. In this regard it is more common to observe non-IgE-mediated manifestations, the treatment of which will not be touched upon in this study. Although strongly controversial, a milk and dairy product exclusion diet in the nursing mother has been suggested.81
- 2.
In cases of atopic dermatitis in infants receiving exclusive maternal breastfeeding, there is no evidence of clinical improvement as a result of the introduction of an exclusion diet on the part of the mother.82,83
- 3.
Nursing mothers with a cow's milk and dairy product exclusion diet should receive supplements in the form of one gram of calcium a day.84
- 1.
If an infant that is breastfed without problems develops symptoms with the introduction of adapted cow's milk formulas, breastfeeding should be continued without the need for the mother to adopt an exclusion diet. It is advisable to maintain breastfeeding for at least 6 months.
- 2.
If the infant requires supplementing or is fed an adapted cow's milk formula, a substitution formula with demonstrated efficacy in the management of CMPA should be provided.85,86
An ideal substitution formula should offer the following properties:
- a.
Safety, with no cross-reactivity with cow's milk proteins
- b.
Adequate nutrition for the infant
- c.
Good palatability
- d.
Facilitation of the acquisition of tolerance
- e.
Low economic cost
- •
Milk from other mammals and unmodified soya, as well as non-adapted rice milk are contraindicated, since they do not meet the necessary metabolic requirements.87 The milk of other mammal species available in this country (e.g., goat and sheep) often exhibit clinical and immunological cross-reactivity with cow's milk proteins.88
- •
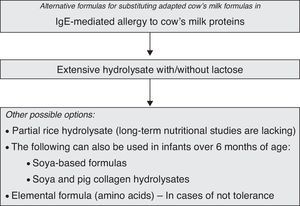
The following treatment options are available for infants with CMPA (Table 1):
- a.
Extensively hydrolyzed formulae (eHF) of cow's milk proteins, which may comprise casein (eHFcas) or serum proteins (eHFsp). Some of these products include fat component modifications with the provision of medium chain triglycerides (MCTs). They may or may not contain lactose as a carbohydrate source. Very extensive hydrolysates contain MCTs and do not contain lactose. These products are called semi-elemental formulas.
- b.
Milk derived from purified soya proteins.
- c.
Milk derived from partial rice protein hydrolysis.
- d.
Milk containing extensive non-dairy soya and meat (pig collagen) protein hydrolysates.
- e.
Elemental free amino acid formulas.
Table 1.Substitution formulas for the dietetic treatment of allergy to cow's milk proteins.
A.- Extensively hydrolysed cow's milk protein formulas•With medium-chain triglycerides and without lactose:ALFARE (S), BLEMIL PLUS FH 1 and 2 (C), DAMIRA (C), DAMIRA 2000 (C), DAMIRA ATOPY (C), NIEDA PLUS (S), PREGESTIMIL (C), PEPTINAUT JUNIOR (S), ALMIRON HIDROLIZADO (S)•Without medium-chain triglycerides and without lactose:NUTRAMIGEN 1 LGG and 2 LGG (from 6 months of age) (C),NUTRIBEN HIDROLIZADA 1 and 2 (C)•Without medium-chain triglycerides and with lactose:ALMIRON PEPTI 1 and 2 (S), LACTO DAMIRA 2000 (C), ALTHERA (S)Protein source: casein (C), milk serum proteins (S)B.- Soya-based formulas•ALSOY, BLEMIL PLUS SOJA 1 and 2, ISOMIL, MILTINA SOJA,NUTRIBEN SOJA, NUTRISOJA, SOM 1, SOM 2, VELACTIN,ALMIRON SOJA, VELACTIN 3 CRECIMIENTO (from 12 months of age)•Soya hydrolysate (containing pig collagen):PREGOMIN0–12 months of age: PEPTIDE, MCT PEPTIDEOver one year of age: PEPTIDE 1+, MCT PEPTIDE 1+C.- Elemental amino acid formulasDAMIRA ELEMENTAL, NEOCATE, NEOCATE ADVANCE,NUTRI 2000 JUNIOR, NUTRAMIGEN AA, ALFAMINO, ALMIRON AAD.- Rice formulasBLEMIL PLUS ARROZ HIDROLIZADO 1 and 2(small amount of soya lecithin)DAMIRA ARROZ HIDROLIZADONOVALAC ARROZ HIDROLIZADOLatest review July 2014 - a.
- •
The treatment of choice in infants with CMPA is a hypoallergenic extensively hydrolysed formula containing peptides with a molecular weight of under 3000Da,89 although most have molecular weights of under 2000Da and even 1000Da. By definition, hypoallergenic formulas should be tolerated by at least 90% of all infants with allergy to cow's milk,90 although this criterion has only been demonstrated for some concrete extensive hydrolysates,91 and allergic reactions have been reported in children extremely sensitive to cow's milk,92 since these hydrolysates can contain small amounts of allergenic proteins.93
- •
The milk response thresholds are highly variable in the population, and their distribution does not follow a “normal” pattern. In effect, some children are extremely sensitive to very low doses of milk proteins, there having been reports of adverse reactions with amounts as low as 0.15–35.8μg.94 A statistical model has estimated that one out of a million individuals exhibits a clinical response threshold dose of 5μg,95 although in most series the lowest dose capable of triggering symptoms has been shown to be above 100μg of milk protein. Thus, only a marginal population of children with CMPA would develop symptoms as a result of the intake of an eHF.96 The residual allergenicity of these formulations shows a greater tendency to produce gastrointestinal symptoms and other manifestations not mediated by IgE. In these cases, we must switch to a different eHF or to an elemental formula based on amino acids.97
- •
The first months of life are a very sensitive period in which any alteration that may occur and any nutritional measure that may be adopted can have later consequences in the life of the patient.
- •
It has been shown that children with cow's milk allergy are at risk of consuming lesser amounts of nutrients than recommended, and this might affect their growth and development.76,97,98 However, in general, eHF ensure adequate growth, in all cases maintaining the same regimens as in those fed with breast milk.99 Long-term (6 and 10 years) nutritional studies have revealed no differences between children fed an eHF and those fed with breast milk or a cow's milk formula.100,101
- •
Extensively hydrolysed formulas have the inconvenience of being less palatable than formulas based on soya or other plant proteins.102 However, the assessment of palatability has been carried out in adults, since this parameter is difficult to measure in infants. The relevance of the palatability data is therefore hard to establish.
- •
If eHF are introduced early (in the first 6 months of life), acceptance is better than when they are introduced at a later stage.
- •
Lactose favours the absorption of calcium in the intestine and clearly improves the palatability of the formula.
- •
The possibility that infants with CMPA might react to protein traces contained in the lactose added to the formulas has led to the avoidance of lactose intake, although different series have demonstrated no clinical reactivity problems.103
- •
Cow's milk hydrolysates are currently available that contain purified lactose without milk protein traces, and which are safe and effective in the treatment of CMPA.104 These formulas with lactose have better flavour characteristics and may be better accepted by children over 6 months of age.
- •
In cases with coexisting secondary lactose intolerance, particularly in infants suffering important digestive alterations with enteropathy and diarrhoea, initial evaluation of lactose exclusion from the diet is indicated.
- •
Partially hydrolysed formulas offer better palatability and are less expensive than eHF. However, because of the residual allergenicity of the scantly hydrolysed peptides, these products are not indicated in the treatment of CMPA.105
- •
Soya-protein based formulas have been used in children with CMPA due to their lesser cost, superior palatability and better acceptance than hydrolysed formulas. The proteins are obtained from the purified extract of this plant product without a hydrolytic stage; they therefore retain their antigenic capacity, and these formulas consequently cannot be regarded as hypoallergenic – although they are tolerated by most patients with CMPA. The problem of soya allergy, widely cited in the Anglo-Saxon literature, has not been evidenced in Spain, where only 4% of the evaluated infants showed co-sensitisation to soya, without clinical expression, and with tolerance in exposure testing in all cases.13
- •
The use of soya is not recommended as a substitute in infants with CMPA under 6 months of age,106 since it is not adequate from the nutritional perspective, and is not indicated in situations of enteropathy sensitive to cow's milk proteins or in non-IgE-mediated allergies.
- •
The nutritional properties of soya milk have been reviewed by the ESPGHAN Nutrition Committee.107 The protein component in these formulas consists of protein isolated from soya flour, with an amino acid profile characterised by methionine, lysine, proline and carnitine deficiency. Such formulas offer lesser bioavailability of minerals such as zinc, iron and calcium, which a high phytate content that impedes adequate mineral absorption. In addition, they contain large concentrations of aluminium and manganese, as well as phytoestrogens (isoflavones) – the long-term effects of which are not clear. The use of these formulas in the first 6 months of life is therefore not recommended.
- •
Hydrolysed rice protein formulas have recently been introduced on the market as substitute treatment for infants with CMPA. They have been found to have scant sensitising capacity and do not produce adverse reactions.108–111
The nutritional suitability of these formulas has been studied in the context of short follow-up periods ranging from 6 months to 2 years.45,46 A lesser z-score for body weight according to age has been evidenced from between 9 and 18 months versus infants without CMPA.112 A study in a healthy infant population has shown rice protein hydrolysates to result in growth and nutritional and biochemical profiles comparable to those found in infants fed with cow's milk, although always in the context of short follow-up periods.113
Extensively hydrolysed non-dairy protein (soya and meat) formulas- •
Formulas based on extensive soya and meat (pig collagen) hydrolysates can also be used. They are more palatable than other hydrolysates, and are comparatively less expensive. However, few data are available on their clinical effectiveness and nutritional safety.
- •
Amino acid formulas114 are used in cases characterised by serious anaphylactic manifestations, which are rarely seen in nursing infants. The elemental amino acid formula is maintained at least until exposure testing to eHF can be made. The risk of reaction or no response to eHFcas is far below 10% of the infants with CMPA, and is more common in patients with multiple allergies.115–117
- •
The use of such formulas can be considered when eHF are rejected by the patient because of palatability problems, since elemental formulas are less bitter than extensively hydrolyzed formulations.
- •
The available amino acid formulas are described in Table 1.
- •
Elimination diet referred to milk and dairy products including yoghourt, cheese, puddings, butter, cream, rice with milk, etc.
- •
Individualised dietetic and nutritional controls are sometimes needed to ensure an adequate intake of proteins, calcium and vitamins A and D, with periodic monitoring to make sure that growth is normal for the age of the patient.118–120
- •
The patient, family and school setting should receive education and training in measures to avoid cow's milk and dairy products, and to adequately deal with any possible adverse reactions.121
- •
Close vigilance is required, carefully checking the labelling of processed foods. In this regard, according to Spanish Royal Decree 1245/2008, of 18 July, and European Union (EU) regulation 1169/2011 of the European Parliament and of the Council of 25 October 2011, specifying the list of allergenic ingredients of mandatory statement, all products containing cow's milk or dairy products must by duly identified.
- •
Cow's milk proteins can receive different denominations: milk as such, sodium caseinate, calcium caseinate, potassium caseinate, magnesium caseinate, protein hydrolysate, casein, milk serum, H4511, H4512, lactoalbumin, or lactoglobulin. A number of websites offer information on the presence of milk in different foods and other common consumer products (www.seicap.es/familiares.asp).
- •
Many prepared foods and products containing cow's milk are sold in fast food outlets, bakeries and restaurants, and their ingredients are sometimes not easy to identify. Cross-contamination with milk is moreover much more likely in such cases.
- •
It is not necessary to eliminate beef from the diet, for although sensitisation is observed in almost 30% of all patients with CMPA (due to the presence of bovine seroalbumin), consumption is tolerated by these individuals without problems.122
- •
The introduction of complementary feeding should not be delayed.
- •
In infants extremely sensitive to cow's milk there have been exceptional reports of allergic reactions after diphtheria–tetanus–pertussis vaccination,123 probably as a result of contamination with milk proteins, as well as with the Sabin vaccine for poliomyelitis.124
- •
Some medicinal products containing as excipient lactose contaminated with cow's milk proteins, and certain probiotic products, may contain significant amounts of milk proteins – with the risk of triggering adverse reactions generally in highly sensitised patients.125–128
The immune response of the gastrointestinal mucosa involves stimuli from the intestinal microbiota, which interact with toll-like immune receptors that play a key role in the development of tolerance, but with effects that are specific of each strain.129 Laboratory studies have shown that supplementing with probiotics, together with the administration of low doses of antigen, favours tolerance.130
There is controversy as to whether supplementing eHF with certain probiotics is able to accelerate the acquisition of tolerance.131,132
The proposed therapeutic feeding algorithm for infants with CMPA is shown in Fig. 2.
Oral immunotherapy (OIT)The current treatment of food allergy involves elimination of the causal food from the diet, avoiding any accidental contact, and using drugs to treat the consequences of any contact that might occur.
Oral immunotherapy (OIT) is an emergent active treatment for inducing tolerance to milk, improving the quality of life of the patients and their families. It consists of administering the allergen (milk) via the oral route, starting with minimum amounts provided on a regular basis, and gradually increasing the amount until reaching the normal target dose for the age of the patient or the maximum tolerated (threshold) dose. The subsequent maintenance doses are administered at home. The aim is to establish immune tolerance, re-educating the complex immune cellular and humoral mechanism in order to correct an inadequate reaction through progressive increments in the amount of allergen ingested.133 Although there have been isolated reports in the literature on the use of OIT in application to food allergy over the last 100 years, most of the investigations on OIT as a new treatment strategy in food allergy have been carried out in the last quarter of a century.134–136 In this regard, OIT has been shown to be effective even in anaphylactic patients,137 being able to modify the immune mechanisms responsible for tolerance138 – although the technique is not without adverse reactions.139
Different protocols have been designed for the usual foods in a number of countries, although no single standardised desensitisation regimen has been defined yet. The different protocols can be classified according to their duration (fast, slow or mixed), the place in which the treatment is provided (hospital admission, partial admission for a few hours, outpatient clinic, home, mixed hospital-home, with weekly or daily increments, etc.), or based on whether premedication is required or not (antihistamines, anti-IgE monoclonal antibodies).
The studies found in the literature generally make no distinction between the effects of OIT versus the natural resolution of CMPA, and do not evaluate permanence of the desensitisation state (tolerance or desensitisation). It should be mentioned that patients who achieve desensitisation through OIT appear to need regular exposure to the allergen in order to maintain tolerance. In contrast, the recurrence of allergy in naturally acquired tolerance is rare. “Desensitisation” refers to the capacity to ingest an allergen without symptoms, but this amount of food allergen must be consumed daily, while “tolerance” refers to the capacity to ingest an allergen without symptoms even after suspending ingestion of the daily maintenance dose. Thus, tolerance does not require maintenance, while desensitisation does require maintenance. The question as to whether these treatments induce only desensitisation or lead to tolerance over the long term is currently being investigated. In the clinical trial published by Staden et al., 75% of the responders who successfully completed OIT reached permanent tolerance after 18–24 months of treatment.140
Once desensitisation has been achieved, the daily intake of a certain amount of milk is advised as maintenance therapy. It has recently been shown that a maintenance regimen involving two weekly doses is as effective as daily dosing (150–200ml), after desensitisation to cow's milk has been achieved with OIT.141
The first double-blind, placebo-controlled study of OIT in patients between 6 and 21 years of age with IgE-mediated CMPA found 92% of the subjects in the active treatment group (12 children) to reach a dose of 5140mg of milk, with no change in tolerance in the control group. The mean incidence of undesired effects was 35% in the active treatment group and 1% in the placebo group. The IgG4 titres increased significantly only in the active treatment group.142
In Spain, Martorell et al.141 found 90% of their series of infants between 2 and 3 years of age to tolerate the maximum dose, versus 23% of the controls. It must be pointed out that many patients experience spontaneous resolution of their IgE-mediated CMPA. Consequently, the risk/benefit ratio of OIT in early infancy must be considered carefully. Adverse reactions are frequent during OIT, although most of them are mild, and none of the patients in the mentioned study suffered serious reactions during desensitisation therapy.
Excellent results have been obtained recently with OIT administered to infants, even under 1 year of age.142 In Spain two groups have documented favourable results with OIT in infants under 1 year of age.143,144
The results obtained have been related to the specific IgE titres at the start of OIT. Higher levels of specific IgE against cow's milk have been correlated to a lesser percentage of children achieving desensitisation and to more serious side effects, in both the induction phase and in the maintenance phase.145
A metaanalysis of OIT in CMPA evaluated five randomised, controlled clinical trials and five observational studies. A total of 218 patients in the controlled trials were found to tolerate 150ml of cow's milk and dairy products, and the probability of tolerance was seen to be ten times greater in the OIT group than in the patients exclusively treated with an elimination diet–thus suggesting OIT to be effective in patients with IgE-mediated CMPA.146 Another metaanalysis showed skin test positivity to the food allergen to decrease significantly, while the specific IgG4 titres were seen to increase, with a substantially lesser risk of reactions to the allergen among those administered OIT.147
The metaanalysis of the Cochrane Collaboration, which analyses all the controlled studies on OIT in application to cow's milk allergy published to date, draws the following conclusions:
- •
Oral immunotherapy is effective for inducing desensitisation in most patients with IgE-mediated CMPA, although the instauration of tolerance over the long term is not clear.
- •
One of the main inconveniences of OIT is the frequency of associated adverse effects, although most of them are mild and self-limiting.
- •
No standardised protocols are available. Adequate guidelines are therefore needed before OIT can be incorporated to clinical practice.148
Omalizumab is a humanised anti-IgE monoclonal antibody being used on an experimental basis to protect patients against the adverse effects that may appear fundamentally in anaphylactic patients or highly sensitised individuals. A number of clinical trials using omalizumab concomitant to OIT have reported good short-term results, although information referred to patient evolution once omalizumab has been withdrawn is still pending.149
Oral immunotherapy is not without adverse effects, and all studies acknowledge the existence of such problems, although they are generally mild. The risk/benefit ratio of this potentially curative treatment modality therefore must be considered. Starting OIT at younger ages may contribute to increase the efficacy of the technique and reduce the adverse effects.
At present, OIT in application to IgE-mediated CMPA should be regarded as a promising treatment that is able to achieve desensitisation in most cases, inducing immune modulating changes, and can promote tolerance. However, the technique must always be used in a centre with experience in the management of OIT and with the capacity to deal with the possible adverse reactions. Additional long-term controlled trials are needed before OIT can be used on a generalised basis in patients with CMPA.
Evolution and prognosis of allergy to cow's milkCow's milk allergy in children generally has a favourable prognosis. In reactions not mediated by IgE, tolerance is achieved in almost 100% of the cases by 2 years of age, while in reactions mediated by IgE tolerance is reached in most cases by about 3 years of age.150–152
Dannaeus and Johansson153 documented the evolution of 47 infants with cow's milk allergy between 6 months and 4 years of age. A total of 29% of the infants with IgE-mediated allergy developed full tolerance to milk in the course of the study versus 74% of the infants with reactions not mediated by IgE. This tendency towards early tolerance in non-IgE-mediated allergy has been observed in most studies.
Hill et al.154,155 in their first study of the natural history of the disease, evaluated the evolution of 47 patients between 3 and 66 months of age with cow's milk allergy confirmed by provocation testing, during a maximum period of 39 months. A total of 38% of the patients acquired tolerance (40% with immediate reactions, 42% with intermediate reactions, and 25% with late reactions). In 1990, these same authors published the 5-year evolution results of a cohort of 100 children with allergy confirmed by provocation testing, and a mean age at the time of diagnosis of 16 months (range 1 month–8 years).3 They found 28%, 56% and 78% of the children to have achieved tolerance after 2, 4 and 6 years, respectively. Of those who reached tolerance, 47% had immediate reactions, 87% intermediate reactions, and 83% late reactions. The last study followed-up on 98 children with cow's milk allergy and a mean age of 2 years (range 6–72 months) (69 with IgE-mediated reactions and 29 with non-IgE-mediated reactions), during a period of 2 years. At the end of the follow-up period, 15/69 (22%) with IgE-mediated allergy versus 17/29 (59%) with non-IgE-mediated allergy had developed tolerance. In the first group, tolerance was associated to lesser specific IgE levels and skin test responses at diagnosis, although some children with tolerance continued to yield intensely positive skin tests.156
James and Sampson60 documented the evolution of 29 children (aged 1 month–11 years) over a 3-year period. A total of 38% developed tolerance, and these patients had lower specific IgE titres at the start, with antibody levels that continued to decrease until tolerance was confirmed.
In Spain, a number of investigators have found most children with IgE-mediated cow's milk allergy to become tolerant in the course of the first years of life.65,157–159 The latest publication, corresponding to a cross-sectional study of 90 children diagnosed with IgE-mediated allergy during the first year of life and followed-up on for a maximum of 7.8 years, used controlled provocation testing to show that 44% were tolerant at 12 months of age, 64% at 24 months, 80% at 3 years, and 86% at 5 years of age. Only 14% were still allergic at 9 years of age.11 Similar results have been obtained in a multicentre study conducted by the Spanish Society of Paediatric Allergology and Clinical Immunology, involving 170 infants with CMPA followed-up on until 4 years of age. Provocation tests were performed at 12, 18, 24, 36 and 48 months of age, and tolerance was recorded in 34% at 12 months, 54% at 18 months, 70% at 2 years, 80% at 3 years, and 83% at 4 years of age.12
Host160 in 2002 published similar findings from the follow-up of a cohort of 1749 Danish children born in 1995. Cow's milk allergy was confirmed in 39/117 children with suggestive digestive symptoms. Of these patients, 45–50% were found to be tolerant at 1 year of age, 60–75% at 2 years, and 85–90% at 3 years of age. In the study conducted by Saarinen et al.161 15% of the children continued to suffer cow's milk allergy at 8.6 years of age.
In 2007, Skripack et al.162 published less satisfactory results: only 19% of 807 children with cow's milk allergy reached tolerance at 4 years of age, 42% at 8 years, 64% at 12 years, and 79% at age 16 years.
The precise mechanisms underlying the development of clinical tolerance to foods are not known, and there are no tests capable of predicting the outcome. In children with food allergy, the disappearance of sensitisation is accompanied by the acquisition of clinical tolerance, with only rare exceptions. However, the instauration of tolerance almost always precedes the loss of sensitisation, and patients who reach tolerance often continue to yield positive skin tests with specific IgE in serum. This situation could reflect maturation of the immune system with an expansion of regulatory T cells. The drop in specific IgE antibody titres coinciding with the exclusion diet, or the increase in specific IgG4 antibodies following regular ingestion, is associated to the instauration of tolerance.
Most studies on the prognostic factors of tolerance agree that the initial specific IgE titres are not predictive of tolerance, although different investigators have recorded higher initial specific IgE levels in children that evolve towards persistent allergy.4,5,7,8,11 A decrease in specific IgE over time is predictive of tolerance, while an increase is a clear indicator of persistent allergy.7,8,11,163 Stripack et al.5 moreover observed that patients with persistent allergy had higher IgE titres in the first 2 years of life (mean 19.0kU/l versus 1.8kU/l; p<0.001) than those who developed tolerance. In general, those individuals reaching higher titres showed a lesser probability of acquiring tolerance.
A number of studies have identified specific IgE against sequential epitopes of casein. In persistent allergy to cow's milk, a greater IgE response against sequential epitopes of α(s1)-casein and β-casein has been described.65,66,164 In particular, IgE against α(s1)-casein may be a predictor of persistence.
In patients with IgE-mediated allergy, most authors have observed a variable association among atopic dermatitis (21–80%),3,14 allergy to other foods (30–78%)2,3,11,13,14 and rhinoconjunctivitis/asthma (30–60%).2,3,5,11,13,14 Studies analysing risk factors for the persistence of cow's milk allergy have described the association to respiratory allergy and/or allergy to other foods as being an indicator of persistence.7–11
The severity of the initial symptoms is not predictive of the evolution towards tolerance.11 However, the persistence of serious symptoms or their appearance over time usually predicts the persistence of allergy. In 2002, Elizur et al.4 found intense skin prick positivity and the severity of the clinical reaction with less than 10ml of milk to be predictive of the persistence of allergy.
Lastly, different studies on the protective role of breastfeeding in the evolution of CMPA have failed to reach firm conclusions.
It is assumed that cow's milk allergy in adolescents and adults is persistent. However, no long-term studies confirming this assumption have been made, and it is possible that new approaches to the treatment of the disease may modify the evolution of cow's milk allergy in the population.
PreventionPrimary preventionConsidering the immaturity of the immune system and the greater permeability of the digestive mucosa in small children, the primary prevention of food allergy has focused on manipulation of the maternal diet during pregnancy and lactation, and of the diet of infants at high risk of allergy (confirmed history of allergic disease in one of the parents and/or siblings) – restricting the intake of foods regarded as allergenic.
There is currently no evidence to recommend mothers to adopt an elimination diet or take supplements such as probiotics during pregnancy and lactation in order to prevent the development of food allergy.82,165–167 In its guide of the year 2000, the American Academy of Paediatrics recommended exclusive breastfeeding until 6 months of age and postponement of the introduction of cow's milk until one year of age, egg until two years of age, and fish and nuts until three years of age.90 However, the results of these recommendations proved disappointing, and in those countries in which they were adopted – such as the United States, Great Britain and Australia – the prevalence of food allergy increased instead of decreasing.168 In 2008, the recommendations were modified on the basis of new evidence, with the conclusion that there are not enough data confirming a protective effect of any dietetic intervention measure from an age of 4–6 months in relation to the development of atopic disease.169
Breast milk is the optimum food in the first 4 months of life, and breastfeeding offers important benefits for both the mother and the nursing infant, but there is little evidence on its preventive effects in relation to the development of food allergy.170
Great importance has been placed on the introduction of adapted formula supplements during the first days of life in the development of cow's milk allergy. However, a randomised, double-blind controlled study did not find early exposure to cow's milk proteins in the first 3 days of life to increase the risk of food allergy.171,172
There is not enough evidence to suggest that formulas containing hydrolysed cow's milk proteins or soya-based formulas are able to prevent cow's milk allergy.173,174
Although the data are contradictory, the clinical experience of the authors indicates that infants receiving artificial feeding in the form of adapted formulas on an uninterrupted basis from birth only exceptionally develop CMPA.13
A recent study has shown that the early introduction of cow's milk proteins from the first 15 days of life as a complement to breastfeeding can promote tolerance, while their introduction at an age of 4–6 months increases the risk of allergy.175 The idea of a protective effect of the early oral introduction of proteins was suggested by Jarret almost 30 years ago.176 The introduction of large amounts of cow's milk proteins from birth and their posterior uninterrupted administration appear to stimulate the induction and maintenance of tolerance, and thus prevent the appearance of cow's milk allergy.
There is not enough evidence to suggest that formulas containing hydrolysed cow's milk proteins or soya-based formulas can prevent cow's milk allergy,173,174 and in any case the early introduction of cow's milk proteins could favour tolerance, as has already been commented above tolerance.
Supplementing the infant diet with prebiotics and probiotics with a view to preventing food allergy has not been shown to offer benefits.177,178
Secondary preventionIn infants at a high risk of suffering allergic disease or with atopic dermatitis fed with breast milk, skin testing with cow's milk can help identify those patients that will experience an adverse reaction when cow's milk is introduced in the diet. If a positive skin test is recorded, controlled provocation testing should be performed before introducing cow's milk in the diet of the infant.
Dietetic recommendations- •
Mothers should not adopt special dietetic measures during pregnancy or lactation.
- •
In infants with a high risk of allergy the same recommendations as in normal infants without risk are applicable:
- 1.
Exclusive breastfeeding during 4 months.
- 2.
If breastfeeding is not possible, or if supplementary feeding is needed, an adapted cow's milk formula is advised.
- 1.
The authors have no conflict of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.