Pharmacotherapy and immunotherapy are the main treatments for allergic diseases to inhalants.
ObjectiveThis study investigates whether to repeat short cycles of immunotherapy after 3 or 5 years the from interruption of the first therapeutic cycle, lasting 3–4 years, to maintain immune memory in individuals subjected to IST.
Methods and ResultsWe have compared two groups, one of 452 patients who, after the first treatment for 3–4 years of IST, performed a cycle of four months after three and 10 years from the suspension, and a second group of 126 individuals who have performed only the IST treatment for 3–4 years. The best results were obtained in the first group.
ConclusionsThese results are due to the immune system's plasticity, a very important concept in clinical practice.
Allergic diseases to inhalants are treated with pharmacotherapy and immunotherapy.1,2 Contrary to pharmacotherapy, the immunotherapy with allergens is the only way to obtain long-term benefits which can persist until 3–5 years after discontinuation of therapy.3 The IST (immunotherapy specific therapy) expected a therapeutic cycle lasting 3–4 years; it is possible to repeat a short cycle to maintain immune memory after 3 or 5 years.
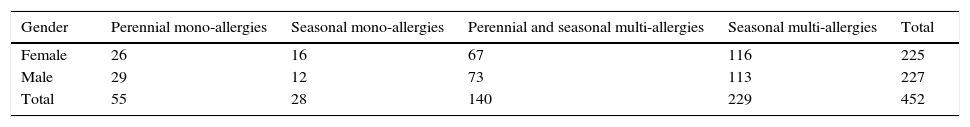
Materials and methodsBetween 1998 and 2000 we selected a group of 452 patients, 6 and 45 years old, divided according to Table 1.
A second group of 126 patients, 6 and 45 years old, enrolled between 1998 and 2000, were compared with the first and divided according to Table 2.
For each patient, after signing the informed consent, we performed PRIST, RAST and prick test for inhalant, IgE Tot., CBC with formula, proteinaemia with Protidogramma, Tryptase4–6 and, in some cases, α-Tryptase and β-Tryptase (Tables 3 and 4).
These patients have been vaccinated for 3 or 4 years (timing determined according to the dosage in the blood of Tryptase and Prick test two years after the start of desensitisation therapy).
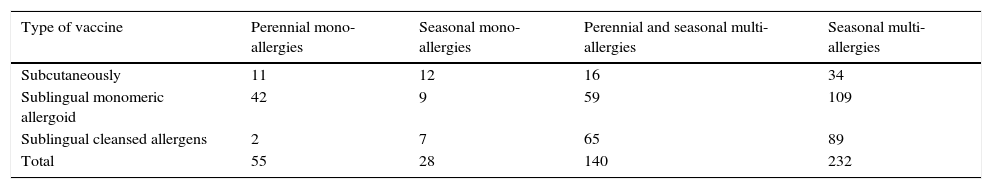
In agreement with what is written by Passalacqua and Bettoncelli we used both the desensitising subcutaneous therapy and SLIT.7–9 We have preferred the latter in most cases, as can be seen from Tables 5 and 6.
Patients of the 1st group classified by type therapy ITS.
| Type of vaccine | Perennial mono-allergies | Seasonal mono-allergies | Perennial and seasonal multi-allergies | Seasonal multi-allergies |
|---|---|---|---|---|
| Subcutaneously | 11 | 12 | 16 | 34 |
| Sublingual monomeric allergoid | 42 | 9 | 59 | 109 |
| Sublingual cleansed allergens | 2 | 7 | 65 | 89 |
| Total | 55 | 28 | 140 | 232 |
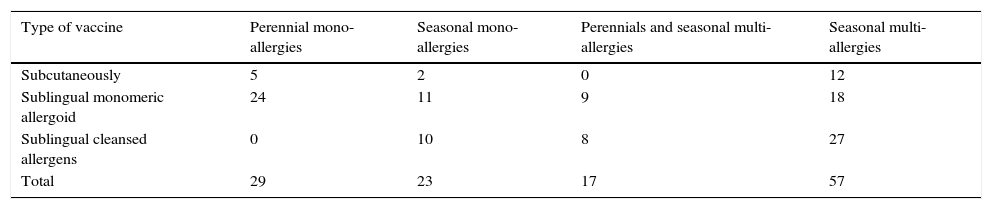
Patients of the 2nd group classified by type therapy ITS.
| Type of vaccine | Perennial mono-allergies | Seasonal mono-allergies | Perennials and seasonal multi-allergies | Seasonal multi-allergies |
|---|---|---|---|---|
| Subcutaneously | 5 | 2 | 0 | 12 |
| Sublingual monomeric allergoid | 24 | 11 | 9 | 18 |
| Sublingual cleansed allergens | 0 | 10 | 8 | 27 |
| Total | 29 | 23 | 17 | 57 |
The patients of both groups were monitored for 14 years, every three months, by visiting ENT, Rhinomanometry, Spirometry and in 22 cases rhino-fibro-scopy. In the study we recruited only those patients with an improvement in their condition equal to or greater than 70%; this was done using a control system with closed questions administered to the patients, the reduction of the drugs used in the period state at least 1/3, the reset of the normal timing of the nasal muco-ciliary transport, to return to acceptable spirometric values, leading to a decline in asthma attacks in those who suffer, and to return to normal level of Tryptase.10–12
This strange number of patients is because they were the ones who remained constant in the quarterly visits, we discarded subjects who did not have adequate compliance.
After three years from the suspension of the immunising therapy, patients of the first group performed SLIT therapy with the same allergens to which they had been desensitised to the full dose, for four years. The same procedure was repeated after seven years from the latter. The patients of the second group had only performed the desensitisation therapy for 3–4 years.
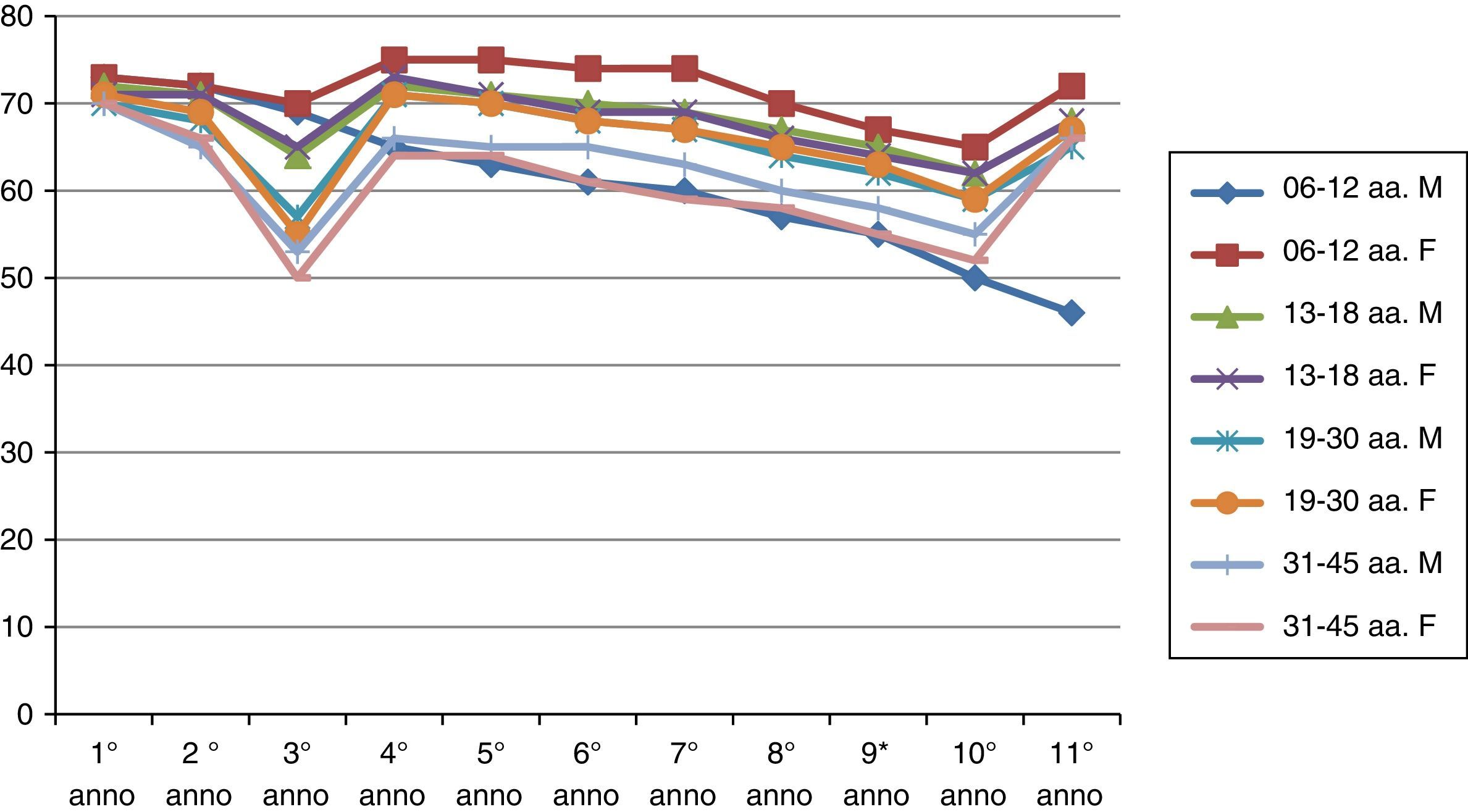
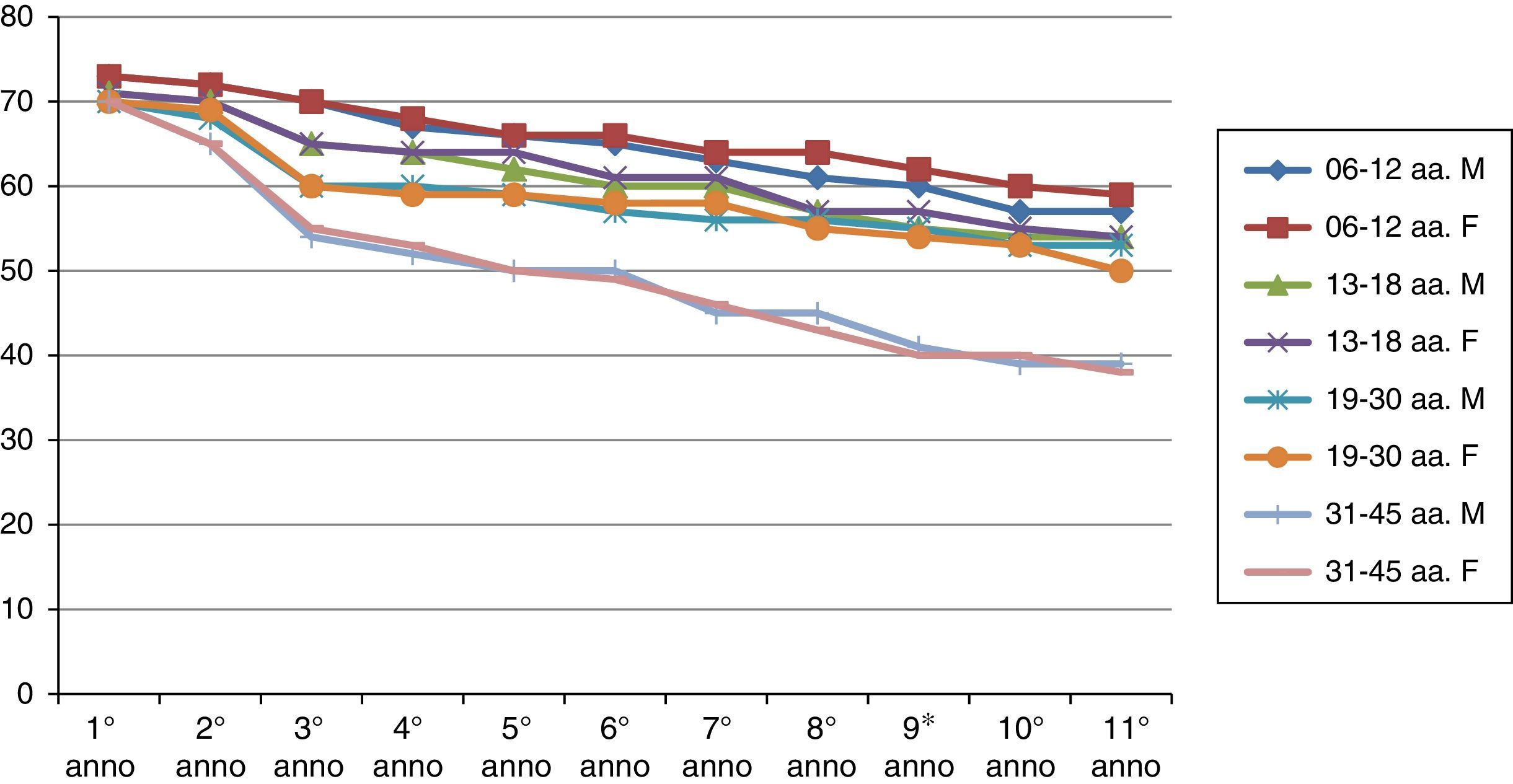
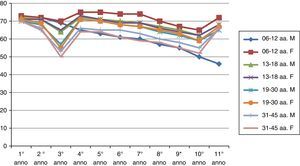
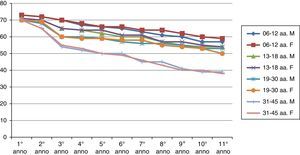
ResultsThe results of the two groups on the basis of the years spent by the interruption of the ITS therapy are shown in the diagrams. The data, of course, are mediated by the percentage of those who belong to bands of the group, thus given the higher number of patients in the first group, the curves are much more similar for the age range (Figs. 1 and 2).
As can be deduced from the figures, all 578 patients treated have reported a reduction in respiratory symptoms, conjunctivitis and a lower use of anti-allergic drugs (antihistamines, both local and systemic corticosteroids), also supported by subjective satisfaction expressed in questionnaires.13–15
It should be remembered that the patients enrolled in this study have had an improvement of their symptoms of above 70%. Moreover, the reduction of respiratory disorders (rhinitis, asthma) was more significant than ocular symptoms.16
The analysis of the data, however, allows us to notice the following key elements:
- 1.
There were no significant differences in the temporal behaviour of the immune induction with subcutaneous and SLIT treatment.17–19
- 2.
Over time, the immunity acquired through ITS tends to decrease, and also remarkably.20,21
- 3.
The immunity acquired from the first group is longer than that of the second group.
- 4.
There is a real difference in the immune behaviour between men and women over time.
- 5.
Early vaccination tends, under equal conditions, to induce longer lasting immunity in time (noticeably in the second group).22–24
The “biological plasticity” describes the ability of a system to change in response to external stimuli. This enables different and appropriate reactions which can exhibit “memory”; the system, after the event, may or may not return to its previous state. The genetic makeup certainly plays a crucial role in this type of susceptibility, although above all the immune system's plasticity is important.25,26
Thus, examining the immune system of different subjects we have found significant differences in similar behaviours; can we explain this as an expression of genetic differences or is it something else?
Analysing the immunity acquired through ITS therapy, we observe that genetics has a minor role, while the most important factors are the environment and individual exposure. The immune system is remarkably plastic in the first years of life, as shown by the results obtained in the vaccination of the first age group (6–12 AA). Until 20 years of age, the immune system is in the process of maturation, it is therefore able to adapt to the most varied environmental conditions and, therefore, to have a better responsiveness to immune induction. After all, a healthy immune system continuously adapts to its encounters with pathogens, harmless microbes that dwell in the intestines, components from the diet and so on; in this case inheritable factors move to the background.
Specific immunity has to be able to answer to all possible molecular combinations in nature and to interact with the organism. Since it is estimated that the number of these combinations is around 10, adaptive immunity should have an equally vast number of cell structures, able to bind specifically to each individual antigen. Since the human genome includes on the whole only 30,000 genes, it is impossible that each antigenic presentation and recognition structure is encoded by a single gene. This paradox can be resolved by the molecular composition analysing the antibodies, of the T lymphocyte receptors (TCR) and the MHC complex: they are protein complexes formed by the combination of structures encoded by multiple (but still numerically limited) variants of genes of the same type. Every cell in the adaptive immune system during its maturation is submitted to a random rearrangement of the genetic repertoire, inherited from the subject in germline; so a unique combination of MHC, TCR antibodies is generated.27–29
Knowing the random rearrangement of genome, what is happening in the vaccinated subjects?
The mechanism which leads to the diversity of antibody heritage (that is the complex of structures assigned to the antigen recognition) inserts small point mutations in genes which codify the forms of antigen recognition structures. This phenomenon is particularly pronounced in B lymphocytes during late maturation (somatic hypermutation). In fact, each individual develops, from the moment of birth, an immune system based on genetic determinants inherited germline; this immune system presents unique characteristics due to the randomness of recombination events and to the selective pressure of the external environment. Antigenic recognition is not pre-established but is able to evolve with the biological history, so the immune system presents advanced functions normally on superior systems such as the central nervous system.
- •
The immune system has to maintain a memory of antigenic recognition's structures which proved to be efficient responding to previous attacks, which could reoccur.
- •
The immune system has to be “trained” to recognise self from non-self to provide a more efficient answer to aggression and prevent autoimmunity. The control of autoimmunity phenomena is called “tolerance” and take place in primary lymphoid organs (central tolerance) during maturation of immune cells and in the suburbs at the end of this process (peripheral tolerance). The specific immunity maturation takes place mainly during the first year, although in reality continues throughout the individual's life.
All this leads to suppose that the adaptive events of immunity are the result of selective dynamics in somatic cells.
This clearly shows the difference in behaviour on acquired immunity between the two groups. The concept, therefore, that acquired immunity is a manifestation of the body's functional plasticity opens new ground in the application of ITS therapy and thus it leads to support a dose recommendation that tends to repeat over time obtained information.30
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestNo conflict of interest, no funding to declare. No grant or other types of payments were given to anyone to produce the manuscript. All the authors have contributed equally to manuscript.