Asthma is a chronic inflammatory, heterogeneous airway disease affecting millions of people around the world. Dendritic cells (DCs) are considered the most important antigen-presenting cell in asthma airway inflammatory reaction. But whether osteoprotegerin (OPG) mediate RANK/RANKL signaling inhibition influences asthma development by affecting the survival and function of DCs remains unclear. In this study, we assessed the effects of OPG on DCs and asthma.
Material and methodsBALB/c mice immunized with ovalbumin (OVA) were challenged thrice with an aerosol of OVA every second day for eight days. Dexamethasone (1.0mg/kg) or OPG (50μg/kg) was administered intraperitoneally to OVA-immunized BALB/c mice on day 24 once a day for nine days. Mice were analyzed for effects of OPG on asthma, inflammatory cell infiltration and cytokine levels in lung tissue. The expression of RANK and β-actin was detected by Western Blot. DCs were isolated from mouse bone morrow. Cell survival was assessed by cell counting. The content of IL-12 was detected by ELISA.
ResultsResults showed that OVA increased the number of inflammatory factors in BALF, elevated lung inflammation scores in mice. OPG reversed the alterations induced by OVA in the asthmatic mice. OPG inhibited the survival and function of DC via inhibition of RANK/RANKL signaling.
ConclusionsThis research proved inhibition of RANK/RANKL signaling by OPG could ease the inflammatory reaction in asthma, providing new evidence for the application of OPG on asthma.
Asthma is a chronic airway inflammation with a variety of cells and cell components involved and millions of people are affected all over the world.1,2 The character of asthma is partially reversible airflow limitation, airway hyper responsiveness, wheezing, cough, and airway remodeling of variable severity.1–3 The pathophysiology of the murine ovalbumin (OVA) model is more similar to allergic asthma in humans, which reproduces several allergic asthma phenotypes, such as eosinophilic airway inflammation, remodeling, and hyperresponsiveness, as well as increased Th2 cytokines and immunoglobulin E levels. It is distinct from exercise-induced asthma or exercise induced bronchoconstriction, both of which are not primarily Th2 mediated.4
Dendritic cells (DCs) are considered to be the most important cell types of the innate immune system which participate in the progress of allergic asthma. DCs are the most functional professional antigen-presenting cells (APCs), which efficiently ingest, process and present antigens. The presentation of naive CD4+ Th cells and their differentiation into Th1, Th2, and Th17 effector cells and Treg cells are all accomplished by DCs. In that steady state, different types of DCs live in the lungs, lines with the conducting airways. Resident DCs contain CD11b+CD103− cells, plasmacytoid DCs (pDCs) and CD11b−CD103+ conventional DCs (cDCs).5 In humans, myeloid dendritic cells (mDCs) have been implicated in allergic asthma as they trigger and maintain asthmatic airway inflammation.6,7 mDCs are considered pro-inflammatory cells as they prime naive T cells from lung and lymph nodes, which results in Th2 differentiation and subsequent Th2 cytokine production, a key feature of asthma.6,7 To prime T cells, immature DC must develop into mature mDCs, which is characterized by the expression of cell surface markers like CD40, CD80, CD83,CD86, and the major histocompatibility class II (MHC-II).6,7
RANKL is a osteoclast differentiation factor expressed in osteoblast cells by activating cytokines and inflammatory factors, such as prostaglandin E2, tumor necrosis factor alpha, prostaglandin E2, interleukin(IL)-1, IL-17 and IL-6.8 RANKL binds to its receptor RANK, which is expressed on the surface of the osteoclast membrane and participates in the regulation of the survival, differentiation and activation of osteoclasts.9,10 Osteoprotegerin (OPG), which is expressed by osteoblasts, competitively binds to RANKL as a decoy receptor of RANKL to inhibit the binding of RANKL to RANK and therefor inhibits the regulation role of RANK/RANKL.11 Also some other cells like T cells express RANKL to induce osteoclastogenesis. Results from numerous reports showed that the health of periodontal is related to immune response from T cells.12,13
The regulatory mechanisms in DC fate decision are mediated in part by the interactions between DCs and T cells. Although the main role of RANK activated by RANKL is the regulation of osteoclasts, RANK also has the role in regulation of DCs.14,15 RANKL expressed on T cells could bind to RANK expressed on DCs and the RANK signaling could activate NF-κB and JNK pathways in DCs.16 Although RANK signaling activated by RANKL could not up-regulate the expression of costimulatory molecules, it could facilitate DCs to express pro-inflammatory cytokines, including IL-12.17,18 RANK/RANKL signaling could promote DC viability by activation of PI3K and up-regulation of NF-κB and Bcl-XL.19,20
Many experiments have shown that RANKL protects mature DCs and prolongs their survival. As a pseudo receptor of RANKL, OPG could counteract the role of RANKL by binding to RANKL competitively. In this study, we intend to find the role of OPG in asthma with a mouse asthma model, and whether OPG participates in immune tolerance by affecting the development and function of DCs.
Materials and methodsEthical approvalEthical approval was received from the Second Affiliated Hospital of Xi’an Jiaotong University.
AnimalsSix-eight-week-old female BALB/c mice were purchased from the Experimental Animal Center of the Fourth Military Medical University (Xi’an, China). The mice were raised at the SPF level animal culture chamber of Xi’an Jiaotong University, and the treatment of experimental animals followed the standard of animal ethics.
Immunization and antigenic challengeMice were immunized with OVA and then challenged by inhalation of OVA according to the method reported previously.21 OVA is a glycoprotein that is the main component of protein in egg white. OVA could cause allergic reactions in BABL/c mice with hyperreactivity in the airway and IgE hypersensitivity in serum. The mice were randomly divided into four groups with six mice in each group: Control, Asthma, OPG and DXM (Dexamethasone) group. Asthma, OPG and DXM groups were administrated with PBS, dexamethasone and OPG respectively after being immunized with OVA. Mice in the Control group were immunized with PBS instead of OVA and administrated with PBS. Specifically, 2.5mg OVA (Grade V; Sigma Chemicals, St. Louis, MO, USA) and 30.05g Al(OH)3 were dissolved in 10ml physiological saline. Mice were administered 0.2ml 1% OVA/Al(OH)3 via intraperitoneal (i.p.) injection on days 0, 7, and 14 and control mice were administered with physiological saline. On day 21, the DXM group mice and OPG group mice were administered dexamethasone (0.01ml/g) and OPG (1mg/per mouse) via i.p. injection once a day for seven days. The asthma group mice and control group mice were administered physiological saline. In the experimental group, the mice were stimulated by 1% OVA to stimulate 30min (the first 30min was given drug intervention), and the normal control group was replaced with physiological saline for the OVA, once a day for seven consecutive days. The behavior of the mice was observed and recorded. There were six mice in each group.
Bone marrow-derived DC culture and stimulationThe mice were anesthetized and executed, and the femur and tibia were separated by PBS for 4–6 times, until the bone marrow was white. The bone marrow cell suspension was collected in the culture dish, centrifuged at 1500×g for 5min and the supernatant discarded. The ACK buffer was added and mixed well, then left for 2min at room temperature, and then centrifuged at 1500×g for 5min. The cell suspension was transferred to the DC complete culture (RPMI-1640 medium, 10% FCS, 5μg/L rmGM-CSF and 2μg/L rmIL-4), and planted to a culture dish and incubated in an incubator with 5% CO2 at 37°C. On day 6, BMDCs were harvested and stimulated with LPS, and/or OPG.
Western blotProtein was extracted from samples with RIPA, and protein concentration was tested by BCA assay. Protein samples were transferred to PVDF membrane after electrophoresis. The PVDF membrane was than incubated with primary antibodies at 4°C overnight, washed with PBST and incubated with secondary antibodies at room temperature for 2h. After washing with PBST, the PVDF membrane was visualized and the image was captured. Antibodies used were as followed: anti-RANK (sc-59981, Santa Cruz, CA, USA), anti- β-Actin (sc-47778, Santa Cruz, CA, USA), and anti-mouse immunoglobulin G (Santa Cruz, CA, USA).
Collection of bronchoalveolar lavage fluid (BALF)Mice were anesthetized, fixed, the trachea was exposed, a small incision was cut, the sterilized syringe needle was inserted, fixed, and the needle should not be inserted too deeply to avoid injury to the lungs. The trachea was irrigated with saline 2–3 times. When a lot of resistance was encountered, the syringe was pulled out and the lavage solution collected.22 BALF was centrifuged at 250×g for 5min and the supernatant was collected.
Measurement of OVA-specific BALF levels of IFN-γ and IL-4The BALF levels of IFN-γ and IL-4 were measured with enzyme-linked immune sorbent assay (ELISA). A 96-well microtiter plate was coated with 100μl sample to be tested or standard sample. Then 50μl biotin-conjugated rat anti-mouse IFN-γ and IL-4 were added to each well and mixed. The plate was moved to 37°C incubator for 60min. The reaction fluid was decanted, and the plate washed with Wash Buffer five times. The avidin horseradish peroxidase (HRP) solution was added to each well and the OD450 was measured within 30min.
Lung histologyMice were anesthetized and the left lungs were fixed with 10% buffered formalin overnight. The lungs were dehydrated transparency with gradient ethanol and xylene and were embedded with paraffin. Then the paraffin blocks were sectioned for 4μm and the sections were dried at 45°C in incubator. Hematoxylin and eosin were stained and the inflammatory pathology score was calculated according to the standard of Underwood.
Statistical analysisSPSS software was employed to statistics. Each group data were displayed with mean±standard deviation. The difference for each group was detected with single-factor analysis of variance for multiple groups. p<0.05 was considered statistically significant.
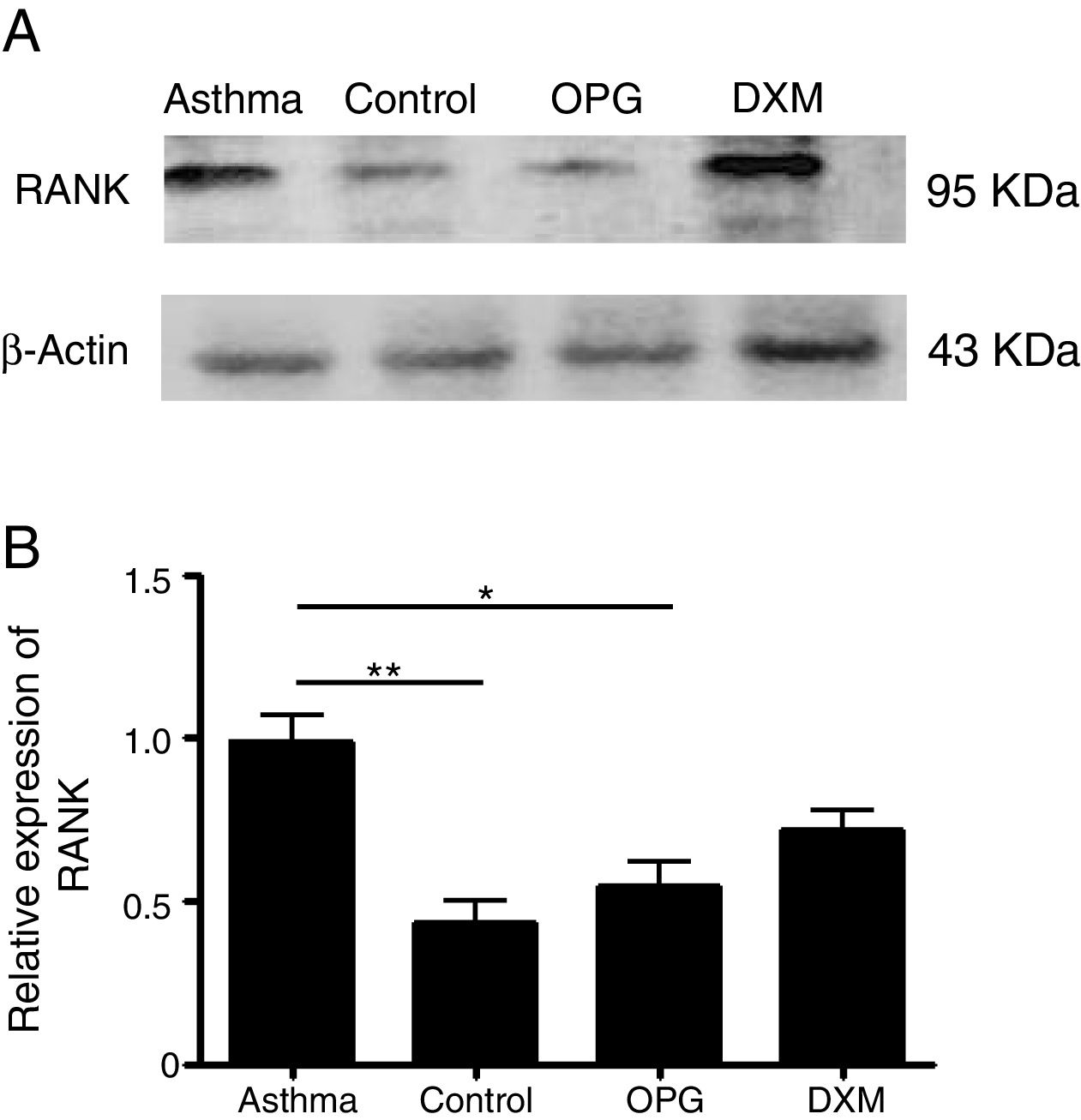
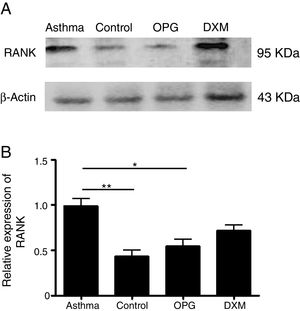
ResultsThe expression of RANK was up-regulated in a mouse model of asthma and OPG down-regulated RANK expressionWe detected the expression of RANK with Western Blot in mouse lung tissues in the four groups: Control group, Asthma group, OPG Group and DXM Group. The results showed that the expression of RANK was up-regulated significantly in the asthma group. OPG effectively down-regulated the expression of RANK while dexamethasone (Fig. 1) did not, suggesting that OPG inhibited RANK/RANKL signaling pathway specifically.
OPG down-regulated RANK expression. (A) The expression of RANK and β-catenin protein level in DCs was detected by Western Blot. (B) The gray scale of band from Western Blot was scanned by Tanon Gis software and the relative value was compared. (*p<0.05, **p<0.01, DXM: dexamethasone).
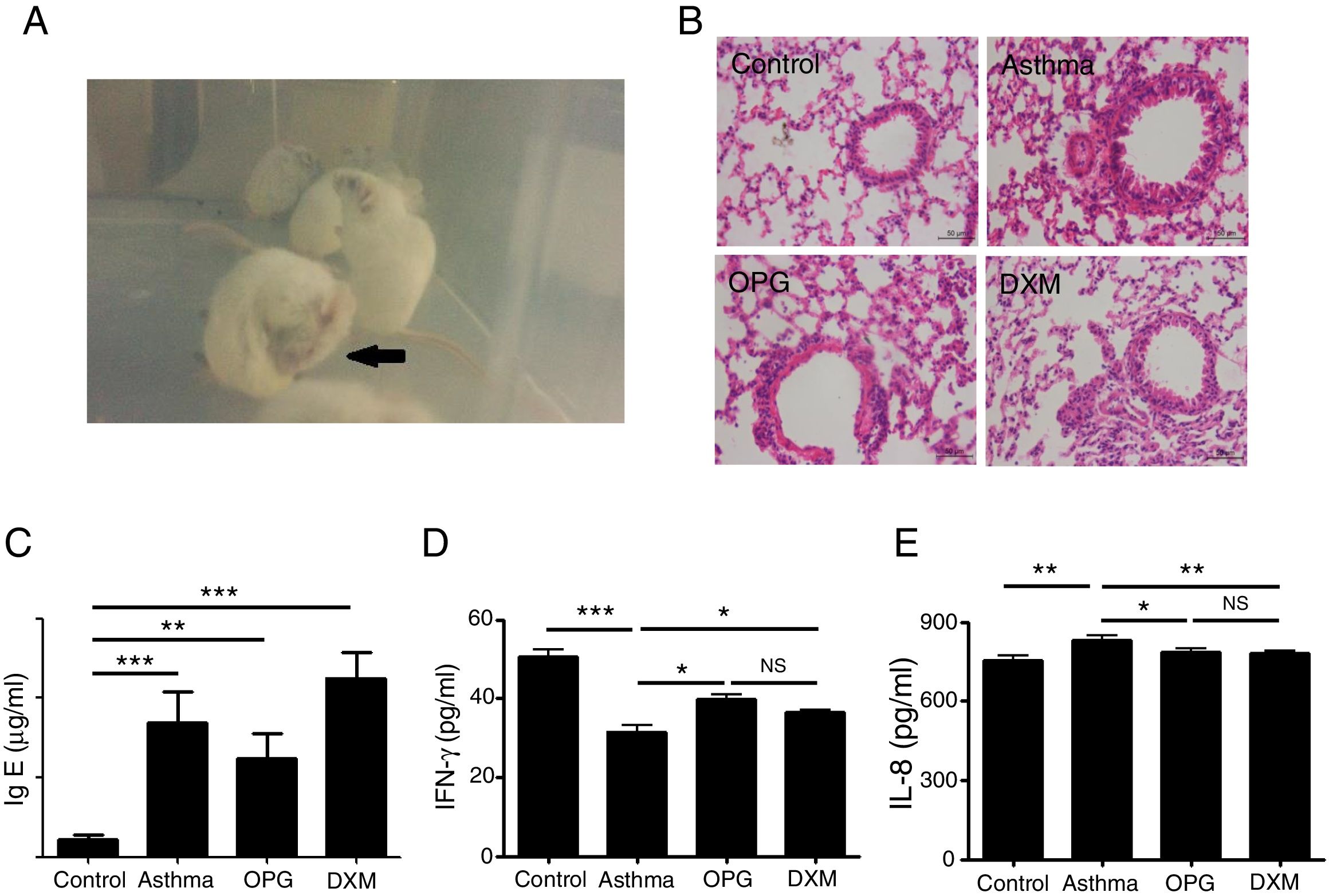
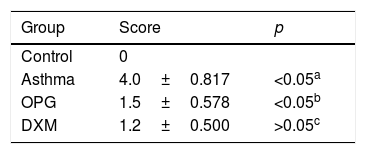
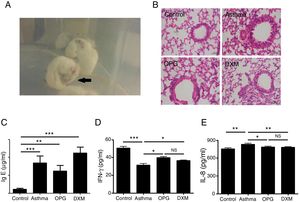
Mice were immunized with OVA to induce the asthma model. Typical behavior was observed after 28 days: scratching head and ears, dysphoria, and accelerated breathing (Fig. 2a). IgE levels from blood serum were significantly higher in the mouse model of asthma than the control (Fig. 2c). Results from hematoxylin and eosin staining showed that the degree of inflammation and inflammatory pathology score of mouse lung tissue in the OPG group and dexamethasone group were alleviated compared with the asthma group (Fig. 2b and Table 1). We also detected the inflammatory factor in BALF. Results showed that IFN-γ of BALF in the OPG group and dexamethasone group was higher than in the asthma group and IL-8 was lower than in the asthma group (Fig. 2d and e). These results suggested that OPG could ease asthma symptoms and inflammatory reactions in a mouse model of asthma mainly through inhibition of RANK/RANKL signaling pathway.
OPG eased asthma inflammatory reaction. (A) Mice were immunized with OVA to induce the asthma model. Typical behavior was observed. (B) Lungs were fixed in 10% buffered formalin, and lung sections were examined by H&E staining. (C) IgE level in blood serum was detected with ELISA and compared. (D) IFN-γ level in BALF was detected with ELISA and compared. E. IL-8 level in BALF was detected with ELISA and compared. (*p<0.05, **p<0.01, ***p<0.001, DXM: dexamethasone).
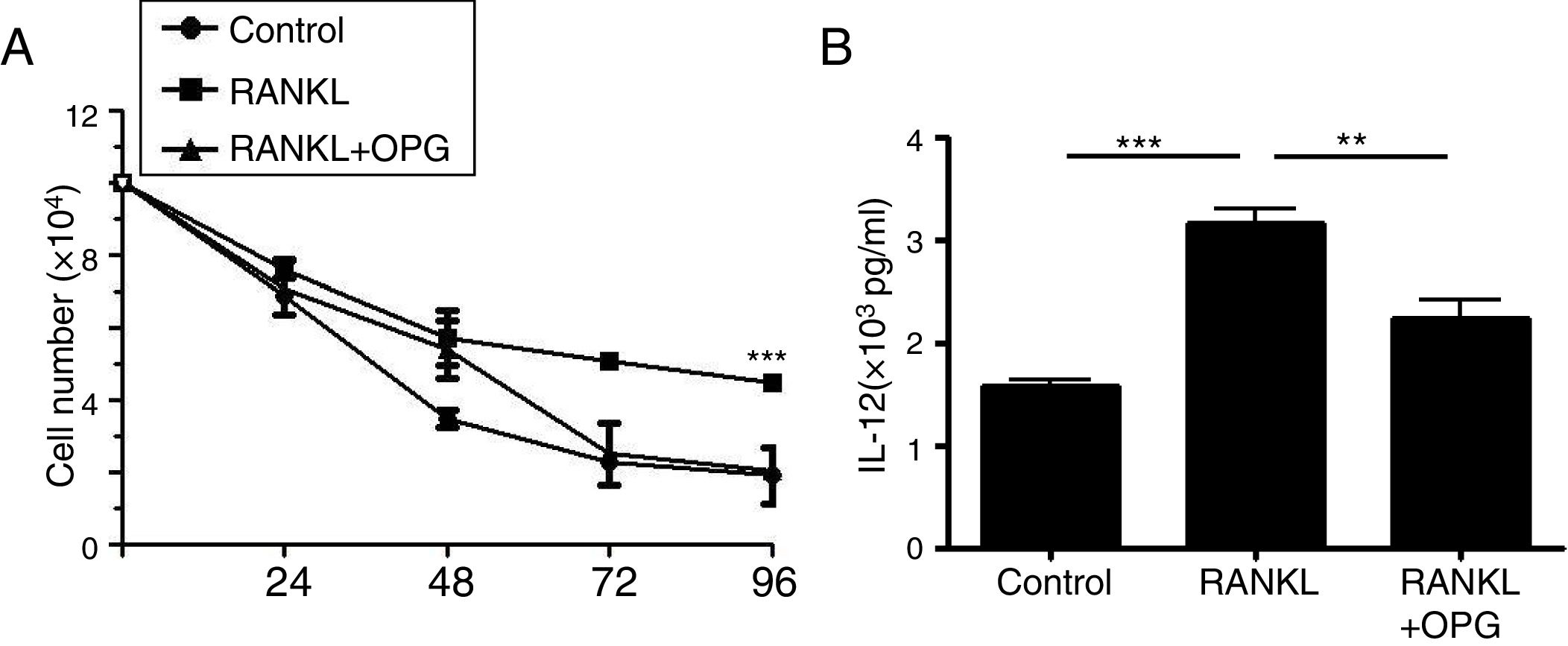
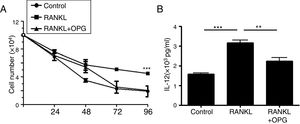
DCs play a vital role in the development of asthma. To explain the mechanism of OPG on asthma and inflammatory reaction, we detected the role of OPG in DCs. The results from cell counting assay showed that activation of RANK/RANKL signaling with RANKL promoted the cell number of DCs, while OPG decreased the number (Fig. 3a). To evaluate the function of DCs, we detected the level of IL-12 in cell supernatant with ELISA. The results showed that activation of RANK/RANKL signaling with RANKL facilitated the secretion of IL-12, while OPG decreased it (Fig. 3b). These results suggested that the RANK/RANKL signaling pathway facilitates the survival and function of DCs, and that OPG alleviates asthma inflammation mainly through inhibition of the survival and function of DCs.
Osteoprotegerin mediate RANK/RANKL signaling inhibition decreased the survival and function of DCs. (A) DCs were treated with PBS (Control), RANKL or RANKL+OPG, and cell numbers were counted after 24h, 48h, 72h, 96h. (B) IL-12 levelin cell supernatant was detected with ELISA and compared. (**p<0.01, ***p<0.001).
Asthma is a chronic respiratory disorder characterized by repeated episodes that can lead to irreversible respiratory arrest.23 In this way, asthma can develop rapidly into complex symptoms. In addition to symptoms associated with airway narrowing, such as shortness of breath, chest tightness and wheezing, airway inflammation is highly sensitive to various factors associated with bronchoconstriction and noxious stimuli. Asthma has different characteristics in different patients, ranging in severity from mild and occasional symptoms to severe and persistent effects on daily life.24 Asthma models have been established in a variety of animals using a variety of methods, with supporters in each animal model – rats,25 dogs,26 rabbits,27 guinea pigs,28 sheep29 and primates.30 However, the most commonly used standard animal model is the OVA-induced mouse asthma model.31 The necessary OVA-induced mouse model does not form true asthma, but produces the same cellular and pathological characteristics as asthma.32
DC is the most powerful APC and plays an important role in primary and secondary immune responses, including allergic immune responses. Mature DCs with high expression co-stimulative molecules CD80 and CD86 activate T cells, while immature DCs with low expression of CD80 and CD86 inhibit T cell response and induce immune tolerance.33,34 Studies showed abnormal activation of DCs in asthmatic patients.35 In this study, we found OPG could inhibit the survival and function of DCs by targeting the RANK/RANKL signaling pathway.
Some researchers reported that RANKL could stimulate DCs to produce pro-inflammatory cytokines, including IL-12.17,18 The RANK/RANKL signaling pathway can activate PI3K by up-regulating Bcl-XL and NF-κB to enhance DC viability.19,20 But whether the RANK/RANKL signaling pathway influences the progress of asthma by regulating the survival and function of DC is unclear.
ConclusionsIn this study, we found that OPG as an inhibitor of RANK/RANKL signaling could ease the inflammatory reaction of asthma by inhibiting the survival and function of DC, provided new evidence for the application of OPG on asthma.
FundingThis work was supported by the grant of a social development research project in the Department of Science and Technology, Shaanxi province, China (The role of curcumin in airway inflammation of asthma mouse and Wnt/β-Catenin signaling pathway of DCs, Grant No: 2014K11-02-03-08; The role of Humulus scandens pollen pTSX pretreatment in airway complaisance inflammation of asthma, Grant No: 2011K12-01-03).
Conflict of interestThe authors have no conflict of interest to declare.
We are indebted to the members of our laboratories at the Department of Respiratory Medicine, the Second Affiliated Hospital of Xi’an Jiaotong University.