Proteus mirabilis poses a critical burden on the breeding industry, but no efficient vaccine is available for animals.
MethodA recombinant Lactococcus lactis expressing the ompA of P. mirabilis was used to develop a vaccine. The mucosal and systemic immune responses of the recombinant vaccine were evaluated in mice after oral immunisation. The inhibition on P. mirabilis colonisation of vaccines was also determined. Moreover, Taishan Pinus massoniana pollen polysaccharides (TPPPS) were used as adjuvants to examine the immunomodulatory effects.
ResultsThe pure recombinant L. lactis vaccine significantly induced the production of specific IgA and IgG, IL-2, IL-4, IFN-γ, and T lymphocyte proliferation, and the immunised mice exhibited significant resistance to P. mirabilis colonisation. Notably, the TPPPS adjuvant vaccines induced higher levels of immune responses than the pure L. lactis.
ConclusionsThe L. lactis as a vaccine vehicle combined with TPPPS adjuvant provides a feasible method for preventing P. mirabilis infection.
Proteus mirabilis is a well-known zoonotic pathogen that widely exists in nature1; this pathogen occurs at an unprecedented rate in animal and human populations and is a major cause of consternation for public health and veterinary communities. P. mirabilis can infect various animals and induce clinical symptoms, such as diarrhoea, sepsis, muscle erosion, and encephalomalacia. The pathogen is also responsible for skin ulcers and causes high mortality and economic losses in aquatic animals.2 In humans, P. mirabilis can cause ascending opportunistic and nosocomial urinary tract infections, which occur in patients with functional or structural abnormalities in the urinary tract.3 In animal husbandry, P. mirabilis leads to serious diarrhoea and death of lambs as well as miscarriage of pregnant sheep in large-scale sheep farms.4,5 The use of antibiotics in controlling P. mirabilis infection has not been recommended because of health hazards to consumers and induced multidrug resistance of pathogenic bacteria. However, few commercial vaccines for animals are available currently. Given the high morbidity rates associated with P. mirabilis infections and the limited therapeutic options, scholars have focused on developing a safe and effective vaccine against P. mirabilis for the breeding industry.
Mucosa tissues are important for protection of an organism from diseases caused by viral, bacterial, and parasitic pathogens, which invade the body through the mucosal system.6 However, vaccines administered by parenteral routes generally fail to induce mucosal immune responses. Therefore, oral vaccination can be an efficient approach for interfering the colonisation of enteropathogenic bacteria; this strategy can effectively induce local immune responses at the intestinal mucosa and concurrently elicit systemic immune responses.7 Nevertheless, orally administered antigens must survive the harsh acidic environment and attack of proteases to interact with the immune tissues of the gut and induce immune responses.6Lactic acid bacteria (LAB) are traditionally used in food industry and generally regarded as safe for human consumption. Lactococcus lactis is a model LAB that has been extensively studied for oral vaccine delivery. L. lactis is used to express some bacterial, viral, and parasitic antigens, and the resultant recombinant strains can induce specific mucosal and systemic immune responses upon oral administration.8–10 In L. lactis expression mode, a Nisin-controlled gene expression system (NICE) can transport the foreign protein to the bacterial cell surface; this mode is an effective and multifunctional tool.11 Thus, oral immunisation with L. lactis carriers exhibits potential for prevention of P. mirabilis infection.
In this study, we constructed a recombinant L. lactis expressing P. mirabilis outer membrane protein A (ompA), one of the main protective antigens in P. mirabilis.12 We also evaluated the specific protection conferred by the recombinant strain against bacterial challenge in mice. Administration of a mucosal adjuvant can augment both mucosal and systemic immune responses to vaccine antigens.
Taishan Pinus massoniana pollen polysaccharides (TPPPS), a pleiotropic polysaccharide extracted from Taishan P. massoniana pollens, has been studied in our laboratory since 2003; TPPPS can be used as an effective adjuvant to improve the immune system and facilitate immune responses.13,14 In the present study, TPPPS was first used as an oral vaccine adjuvant. The effects of TPPPS on conditioning intestine mucosal immunity were also investigated.
Methods and materialsEthics statementThe animal procedures used in this study were approved by the Animal Care and Use Committee of Shandong Agricultural University (permit number: 20010510) and performed in accordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, China).
Bacterial strain, vector, and mediumP. mirabilis strain PM.1 was isolated from a dead lamb with diarrhoea in 2013 (Shandong, China) and then preserved in our laboratory. The plasmid pNZ8149 and L. lactis NZ3900 strain were purchased from MoBiTec GmbH (Goettingen, Germany). Escherichia coli DH5α and pMD18-T were purchased from Takara Co., Ltd., China. Elliker-medium [yeast extract, 5g/L; tryptone, 20g/L; NaCl, 4g/L; CH3COONa, 1.5g/L; L(+) ascorbic acid, 0.5g/L; agar, 15g/L; 2 and bromocresol purple 0.5%, pH=6.8] and M17 broth medium containing 0.5% lactose were purchased from Sigma (Beijing, China). All yeast culture media were prepared in accordance with the manufacturers’ guidelines.
Construction of recombinant pNZ-ompA/L. lactisOn the basis of the ompA gene sequence of P. mirabilis (GenBank Type: RefSeq (Nucleotide) NC_010554.1), a pair of primers (ompA-F: 5′-CCCATGGG TATGATAACGAGGCGTAAAATGAAAAAGACAGCTATCGCATTAGCAG-3′, ompA-R: 5′-CTGCTCTAGATTAGTGACCAGGTTGAACAACAAC-3′) was designed to produce a 1107bp fragment by polymerase chain reaction (PCR). The PCR product was then digested with the restriction enzymes Nco I and Xba I, and the digested fragment was cloned into plasmid pNZ8149. The resultant plasmid was confirmed by sequencing (Sunny, Shanghai) and transformed into L. lactis NZ3900 (named pNZ-ompA/L. lactis). The blank pNZ8149 plasmid was transformed into L. lactis NZ3900 as negative control and named blank-pNZ/L. lactis.
Protein expression, flow cytometry, and immunofluorescence microscopyRecombinant L. lactis NZ3900 cells were cultured in M17 broth medium containing 0.5% lactose as carbon source at 30°C overnight under anaerobic conditions. Recombinant pNZ-ompA/L. lactis cells were diluted by 1:25 to 50 in M17 medium. When the cell density reached 0.4 of OD600, Nisin was added every six hours to ensure continuous induction up to a final concentration of 10ng/mL. Western blot analyses were performed to identify ompA by using methods described in previous study.12 The mouse anti-ompA protein monoclonal antibody used in Western blot analysis was prepared in the laboratory.
The recombinant pNZ-ompA/L. lactis and blank-pNZ/L. lactis were centrifuged at 5000×g for 10min at 4°C and washed thrice with sterile phosphate-buffered saline (PBS) to investigate whether the ompA protein was expressed on the surface of L. lactis. The bacteria were then incubated with mouse anti-ompA protein monoclonal antibody at 4°C overnight, followed by fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Sigma, China). The stained cells were analysed by flow cytometry (Guaga Easy Cyte Mini, USA).
For immunofluorescence staining, the recombinant pNZ-ompA/L. lactis and blank-pNZ/L. lactis were harvested after induction and incubated with mouse anti-ompA monoclonal antibody as the primary antibody followed by FITC-conjugated goat anti-mouse IgG (Sigma, China) as the secondary antibody. The blank-pNZ/L. lactis was used as negative control.
Vaccine preparationThe cultured recombinant pNZ-ompA/L. lactis was resuspended in sterile PBS at a concentration of 1×1011 colony-forming unit (CFU)/mL (100μL/mouse). The corresponding blank-pNZ/L. lactis was treated similarly.
TPPPS was prepared in our laboratory through water extraction and ethanol precipitation.13 The contents of TPPPS were set at the following three doses: 50 (low), 100 (moderate), and 200 (high) mg/mL in three separate TPPPS adjuvant vaccines. The recombinant pNZ-ompA/L. lactis mixed with three doses of TPPPS was separately prepared to obtain the corresponding adjuvant oral vaccine.
Animal experimentA total of 360 six-week-old SPF BALB/c mice (female; Spirax Ferrer Poultry Co., Ltd, Jinan) were randomly separated into six sterilised isolators (groups I–VI), with 60 mice each. The ambient conditions were set to 20–25°C and 30–40% relative humidity. Air entering the isolators was filtered. Mice in groups I–VI were inoculated orally with 0.1mL of low, moderate, and high doses of TPPPS adjuvant recombinant pNZ-ompA/L. lactis, pure pNZ-ompA/L. lactis, blank-pNZ/L. lactis, and PBS. Groups I–VI were named pNZ-ompA-TPPPS (L), pNZ-ompA-TPPPS (M), pNZ-ompA-TPPPS (H), pNZ-ompA, blank-pNZ, and Mock, respectively. The vaccines were inoculated daily at 0–4dpi (days post the first inoculation). Two booster immunisations were conducted at 10–14dpi and 24–28dpi.
At 0, 14, 28, 42, and 56dpi, three mice in each group were selected randomly to determine the antibody titres and the concentrations of IL-2, IFN-γ, IL-4, and interleukin 10 (IL-10) in serum, as well as T-cell proliferative response (LTRs) and counts of CD4+ and CD8+ T lymphocytes in peripheral blood. The tracheal and intestinal lavage fluids were collected to determine sIgA titres by using methods described in previous study.15 The animals were starved for 12h before sampling.
Detection of specific IgG and sIgA antibodies as well as IL-2, IFN-γ, IL-4, and IL-10Three sera, tracheal, and intestinal lavage fluid samples were randomly collected from each group during sampling. Standard enzyme-linked immunosorbent assay (ELISA) protocol was performed for specific antibody IgG titres in serum and sIgA titres in intestinal and tracheal lavage samples.16 The concentrations of IL-2, IFN-γ, IL-4, and IL-10 were detected using mouse IL-2, IFN-γ, IL-4, and IL-10 ELISA kits (Langdon Bio-technology Co., Ltd, Shanghai) in accordance with the manufacturer's instructions. Absorbance was determined with a microplate reader at 450nm.
Counts of CD4+ and CD8+ T lymphocytes in peripheral bloodThe fresh anticoagulant-heart blood was collected, and then the lymphocytes were obtained with lymphocyte separation medium (P8620-200, Solarbio, China) after centrifugation at 2000rpm for 10min. Then, 10μL of fluorescein isothiocyanate anti-mouse CD4 antibody (BioLegend, USA) and 10μL of phycoerythrin anti-mouse CD8 antibody (BioLegend, USA) were decanted into 50μL of lymphocyte suspension. The mixture was incubated for 20min at 4°C.17 The percentages of CD4+ and CD8+ T lymphocytes were detected through flow cytometry (Guaga Easy Cyte Mini, USA) in accordance with the manufacturer's instructions.
Peripheral blood lymphocyte proliferationFresh anti-coagulated peripheral blood samples were collected from the mice selected in each group and used to separate lymphocytes as previously described.18
Challenge with P. mirabilisAt one day after the last immunisation, 20 mice from each group were challenged orally with LD50 of P. mirabilis. The number of P. mirabilis colonies in the intestine was determined. Faecal excretion of P. mirabilis was monitored every two days, and serial dilutions of the samples were plated in blood agar. α-Haemolytic colonies were determined after incubation of the plates for 24h at 37°C.
Twenty mice from each group were challenged with 10 LD50P. mirabilis two weeks after the last immunisation. Mice were maintained for seven days post challenge, and deaths were recorded every day. The survival status of mice was calculated with the following formula:Survival rate (%)=No. of surviving mice/Total No.×100
Statistical analysisData were presented as mean±standard deviation (SD), and Duncan's multiple-range test was performed to analyse differences among groups by using SPSS 17.0 software. A P-value of<0.05 was considered statistically significant.
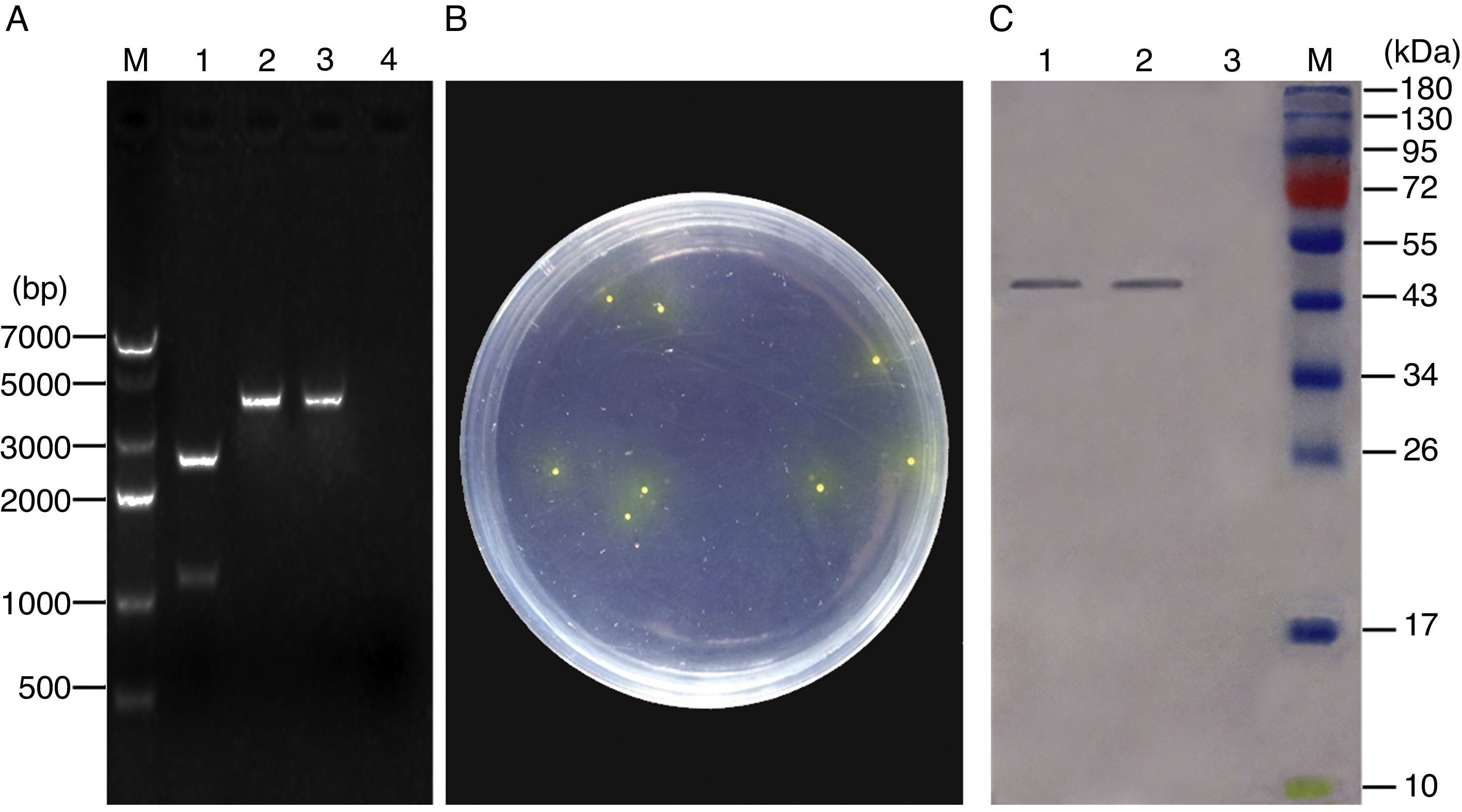
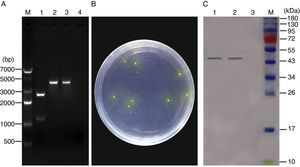
ResultsConstruction of recombinant pNZ-ompA/L. lactis and ompA protein expression in vitroThe recombinant plasmid pNZ-ompA was first constructed. The insertion of the ompA gene into the pNZ8149 plasmid was confirmed by restriction enzyme digestion (Fig. 1A). Analysis of the phenotypic screening of recombinant L. lactis utilising lactose in Elliker medium showed that the recombinant pNZ-ompA/L. lactis presented yellow colonies (Fig. 1B). The molecular size of the target protein was approximately 45kDa. Western blot analysis indicated the lack of the clear band in blank-pNZ/L. lactis used as control (Fig. 1C).
Identification of ompA gene expression in the recombinant L. lactis. (A) Identification of the recombinant pNZ-ompA plasmid by digestion with restriction enzymes. M: DNA 7000bp ladder; 1: the recombinant pNZ-ompA plasmid digested with NcoI and XbaI. The digested ompA gene at the bottom (1107bp); the backbone of the pNZ8149 plasmid at the top (2548bp); 2: the recombinant pNZ-ompA plasmid digested by single NcoI enzyme (3655bp); 3: the recombinant pNZ-ompA plasmid digested by single XbaI (3655bp). (B) Phenotypic screening of recombinant pNZ-ompA/L. lactis utilising lactose in Elliker medium. The recombinant pNZ-ompA/L. lactis presented yellow colony. (C) Detection of ompA expression by Western blot assay. The mouse anti-ompA monoclonal antibody was used in this assay. M: Pageruler pre-stained protein ladder; the predicted bands (approximately 45kDa) of the ompA protein were detected in lanes 1–2. No band was shown in Lane 3 carrying a control protein β-actin.
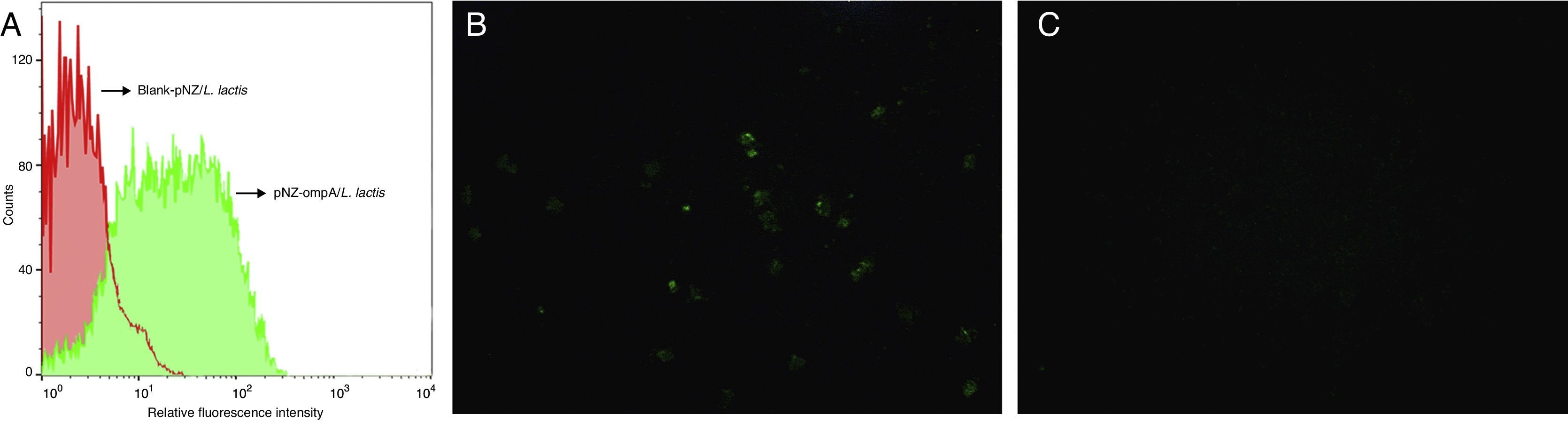
To determine whether the ompA protein was expressed on the surface of L. lactis, we performed flow cytometric analysis. The results showed that the fluorescence intensity significantly increased in the recombinant pNZ-ompA/L. lactis (Fig. 2A). Immunofluorescence analysis further showed that the recombinant pNZ-ompA/L. lactis was immunostained positive for ompA (Fig. 2B), but blank-pNZ/L. lactis did not (Fig. 2C). Hence, the constructed recombinant pNZ-ompA/L. lactis could effectively display the ompA protein on the L. lactis surface.
Flow cytometric and immunofluorescence analysis of the L. lactis expressing ompA. (A) Flow cytometric analysis of the L. lactis expressing ompA. The mouse anti-ompA monoclonal antibody was used in this assay. The recombinant pNZ-ompA/L. lactis showed a significant increase of fluorescence intensity (green); the blank-pNZ/L. lactis showed negative fluorescence (red). (B and C) Immunofluorescence analysis of the L. lactis expressing ompA. The mouse anti-ompA monoclonal antibody was used in this assay. The recombinant pNZ-ompA/L. lactis showed positive green fluorescence on the cells (B). No fluorescence was shown in the blank-pNZ/L. lactis (C).
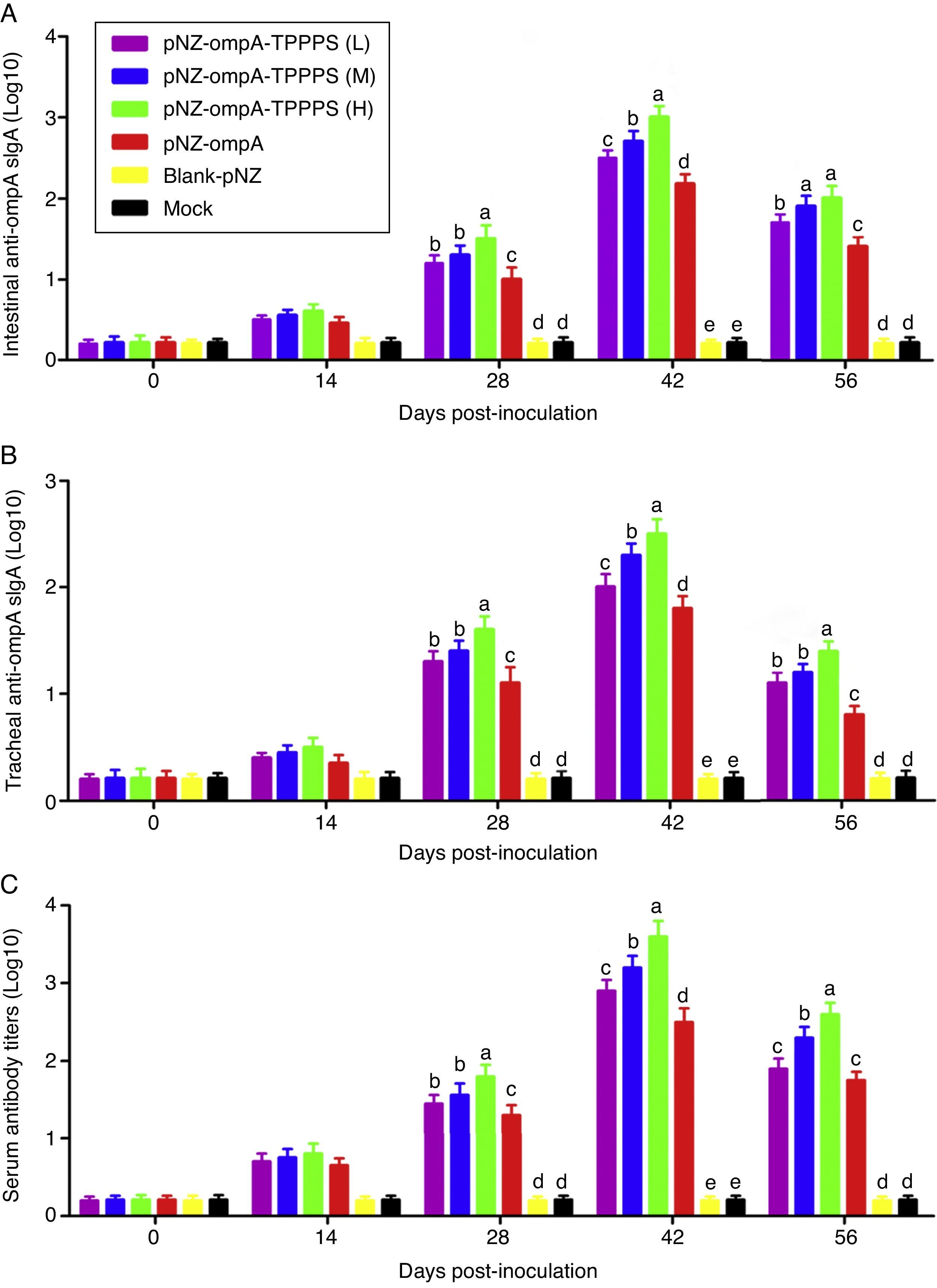
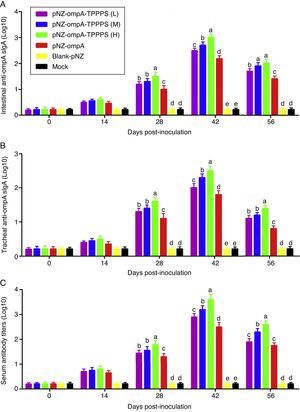
Mucosal antibodies play an important role in protection against pathogens. In this study, sIgA responses in intestinal and tracheal lavage fluid samples were analysed by ELISA. The anti-ompA sIgA levels in the pNZ-ompA group are significantly higher than those in the control groups inoculated with blank-pNZ/L. lactis or PBS in the intestine and trachea at 28–56dpi (Fig. 3A and B; P<0.05). Notably, a significantly higher anti-ompA sIgA response was detected in groups pNZ-ompA-TPPPS (L), (M), and (H) than that in group pNZ-ompA. The group pNZ-ompA-TPPPS (H) produced the highest anti-ompA sIgA titres in the intestine and trachea at 28–42dpi (P<0.05).
Changes in the intestinal and tracheal specific sIgA and serum antibody titres of the mice inoculated with oral vaccines. Mice in six groups were inoculated with 50, 100, and 200mg/mL of TPPPS adjuvant recombinant pNZ-ompA/L. lactis, pure recombinant pNZ-ompA/L. lactis, blank-pNZ/L. lactis, and PBS respectively at 0–4, 10–14, and 24–28dpi. Intestinal and tracheal lavage fluids and serums were collected at 0, 14, 28, 42, and 56dpi. The specific sIgA and serum antibody titres were then determined by indirect ELISA. All values shown are the means±SD of three independent experiments. Different superscripts indicate a significant difference (P<0.05).
Anti-ompA IgG responses were evaluated in serum samples. The results showed that the anti-ompA IgG titres in mice vaccinated with pNZ-ompA are significantly higher than those in the control groups inoculated with blank-pNZ/L. lactis or PBS at 28–56dpi (Fig. 3C; P<0.05). Mice in groups pNZ-ompA-TPPPS (L), (M), and (H) exhibited significantly higher levels of anti-ompA IgG than those that received pure pNZ-ompA at 28–42dpi compared with the TPPPS adjuvant formulations (P<0.05). Group pNZ-ompA-TPPPS (H) showed increased IgG production compared with pNZ-ompA-TPPPS (L) and (M) at 28–56dpi (P<0.05).
These results showed that the constructed recombinant pNZ-ompA/L. lactis can elicit both ompA-specific mucosal and systemic antibody responses via oral inoculation. Additionally, TPPPS promoted antibody titres induced by pNZ-ompA/L. lactis in mice, especially at a concentration of 200mg/mL.
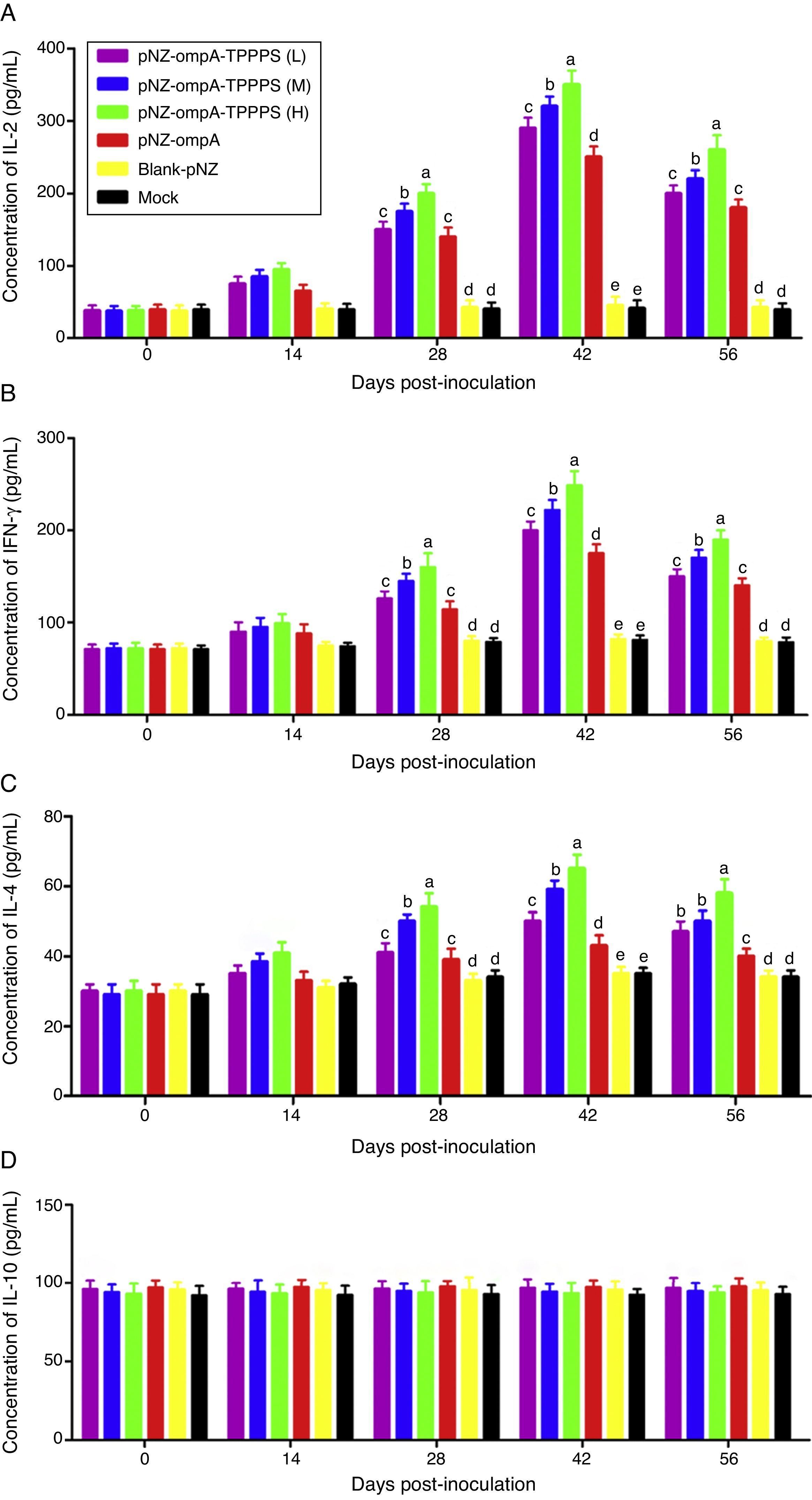
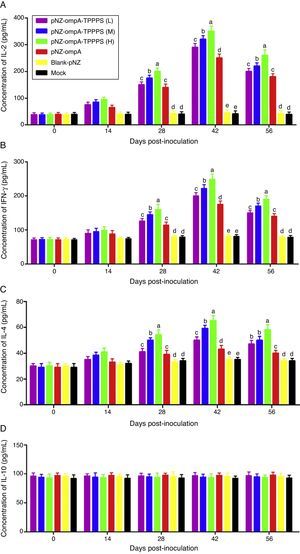
TPPPS promoted cell-mediated immune responses to the recombinant pNZ-ompA/L. lactisCytokines IL-2, INF-γ, IL-4, and IL-10 in serum were determined to characterise cellular immune responses induced by oral immunisation with the recombinant pNZ-ompA/L. lactis. Immunised mice in group pNZ-ompA produced significantly higher IL-2, INF-γ, and IL-4 levels than those inoculated with blank-pNZ/L. lactis or PBS at 28–56dpi (Fig. 4A–C; P<0.05). Moreover, IL-2, INF-γ, and IL-4 secretion was significantly enhanced in groups pNZ-ompA-TPPPS (L), (M), and (H) compared with that in group pNZ-ompA without TPPPS (P<0.05). Notably, group pNZ-ompA-TPPPS (H) showed increased IL-2, INF-γ, and IL-4 levels compared with groups pNZ-ompA-TPPPS (L) and (M) (P<0.05). However, IL-10 production in the six groups showed no significant differences (Fig. 4D; P>0.05).
Changes in cytokines of the mice inoculated with vaccines. Mice in six groups were inoculated with 50, 100, and 200mg/mL of TPPPS adjuvant recombinant pNZ-ompA/L. lactis, pure recombinant pNZ-ompA/L. lactis, blank-pNZ/L. lactis, and PBS respectively at 0–4, 10–14, and 24–28dpi. Serum was collected at 0, 14, 28, 42, and 56dpi. IL-2 (A), IFN-γ (B), IL-4 (C) and IL-10 (D) were detected by using the mouse IL-2, IFN-γ, IL-4 and IL-10 ELISA kits. All values shown are the means±SD of three independent experiments. Different superscripts indicate a significant difference (P<0.05).
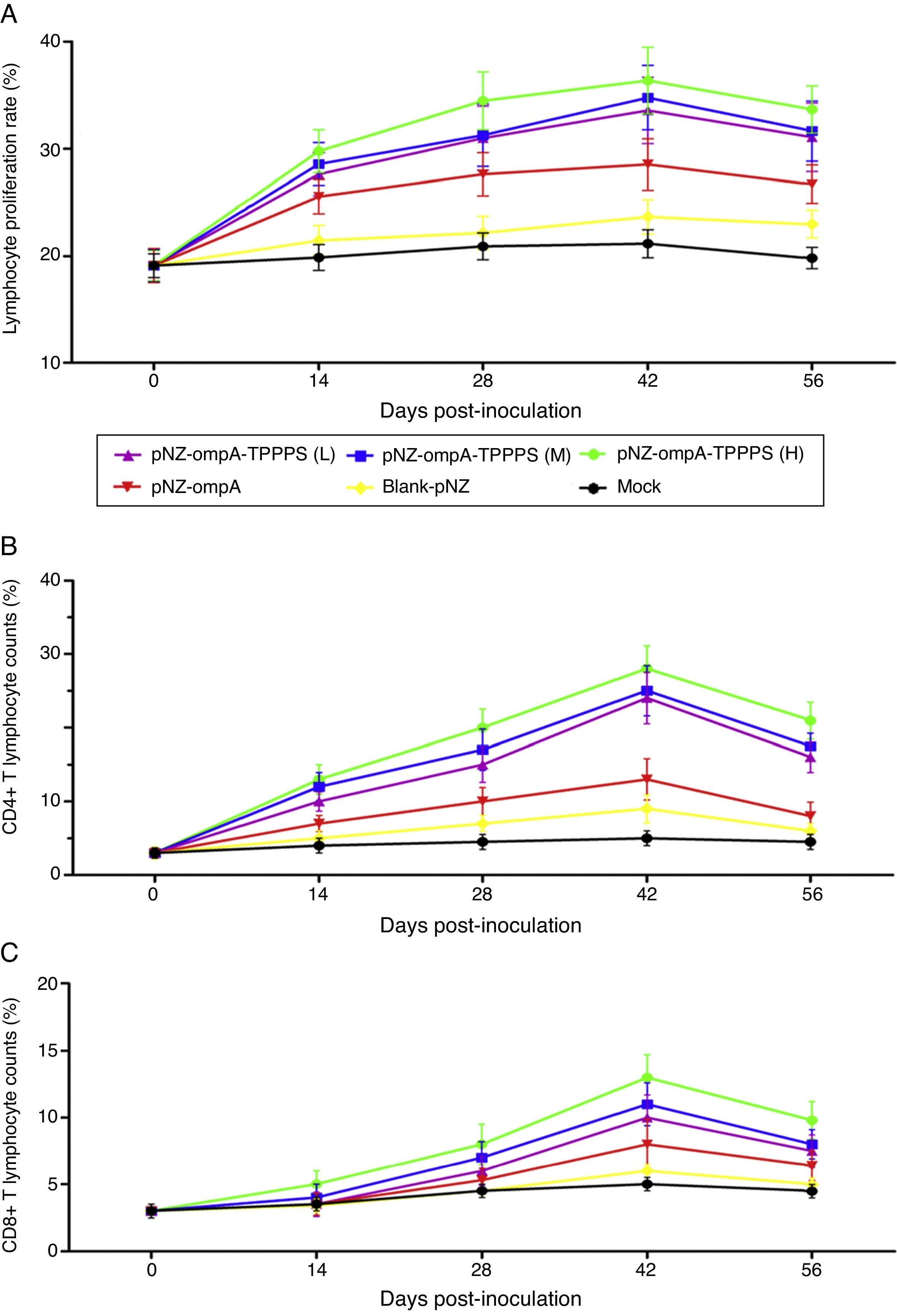
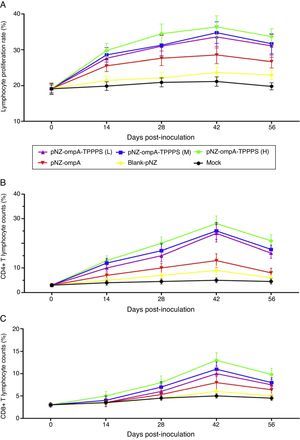
The ratio of lymphocyte proliferation is commonly used to evaluate cellular immunity.19 In the present study, we found that mice in group pNZ-ompA showed significantly higher LTRs than control mice that received blank-pNZ/L. lactis or PBS at 14–56dpi (Fig. 5A; P<0.05). By contrast, the LTRs in mice immunised with TPPPS adjuvant pNZ-ompA are higher than those immunised with pNZ-ompA alone at 28–56dpi (P<0.05). The LTRs were the highest in mice immunised with pNZ-ompA-TPPPS (H) but were not significantly different from those in groups pNZ-ompA-TPPPS (L) and (M) (P>0.05). The number of CD4+ and CD8+ T lymphocytes in serum directly reflects immune function in animals.20 Overall, the counts of T lymphocytes showed similar trends to that of LTRs (Fig. 5B and C). In these two detection indices, the optimal effects were obtained when at 200mg/mL TPPPS was used.
Changes in LTRs, CD4+, and CD8+ T lymphocytes in mice inoculated with vaccines. Mice in six groups were inoculated with 50, 100, and 200mg/mL of TPPPS adjuvant recombinant pNZ-ompA/L. lactis, pure recombinant pNZ-ompA/L. lactis, blank-pNZ/L. lactis, and PBS respectively at 0–4, 10–14, and 24–28dpi. Serum was collected at 0, 14, 28, 42, and 56dpi. Then the LTRs, and the percentages of CD4+ and CD8+ T lymphocytes were detected by flow cytometry. An asterisk indicates that the value of the corresponding group was significantly different from that of the control group (P<0.05).
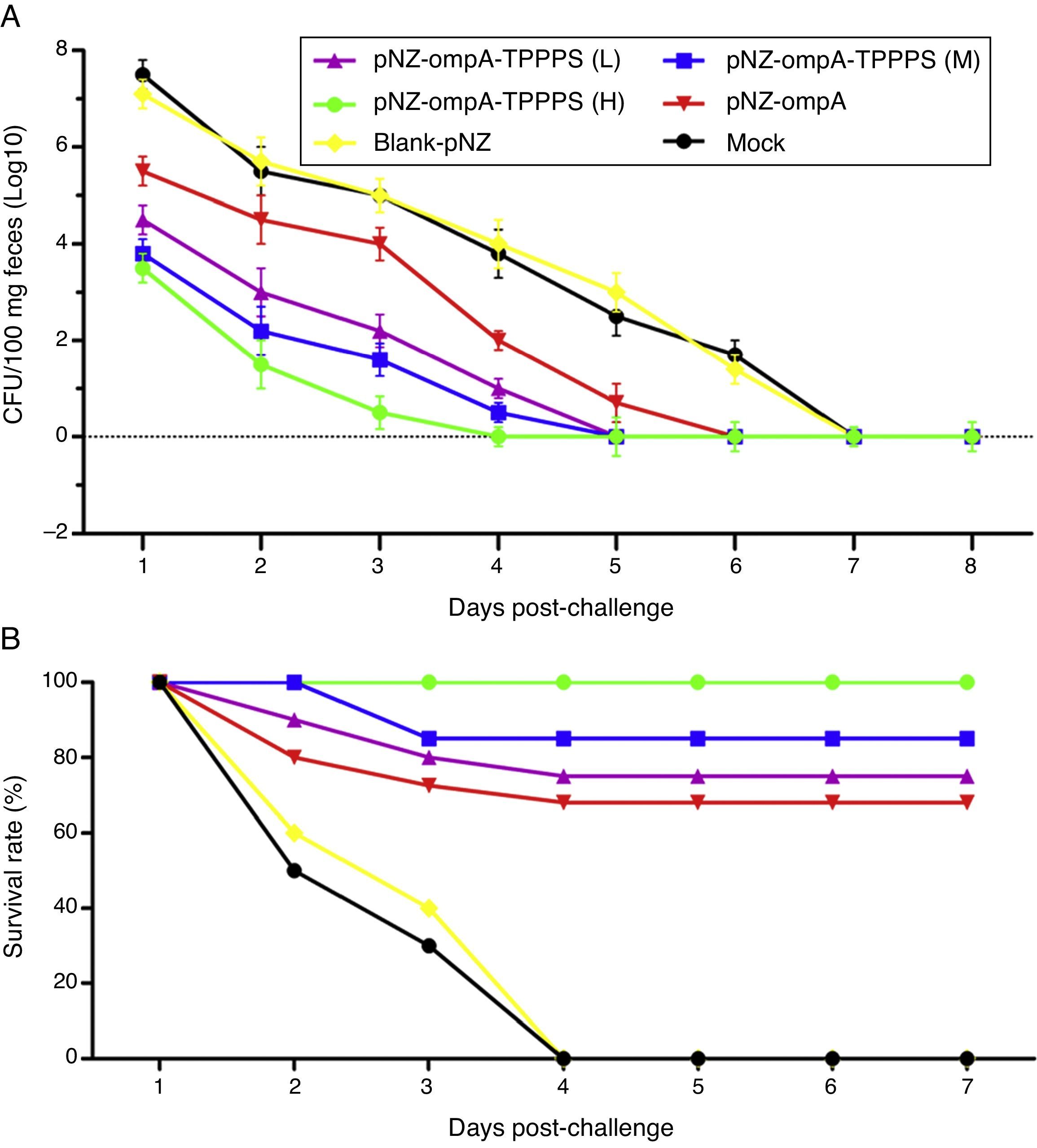
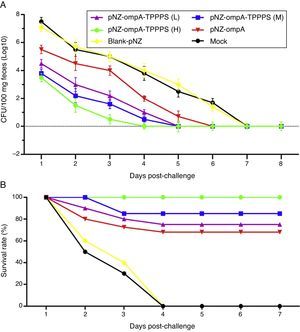
To evaluate the resistance of pNZ-ompA/L. lactis on P. mirabilis colonisation, we challenged the mice with P. mirabilis 1 d after the last immunisation. P. mirabilis colonisation was significantly reduced in the small intestine in group pNZ-ompA compared with those in groups blank-pNZ and Mock (Fig. 6A; P<0.05). Interestingly, the administration of TPPPS adjuvant pNZ-ompA conferred improved inhibition on P. mirabilis colonisation than pNZ-ompA administration alone (P<0.05). Group pNZ-ompA-TPPPS (H) showed the highest resistance to P. mirabilis colonisation (P<0.05).
Intestinal colonisation and protective rates of P. mirabilis-challenged mice. Mice in six groups were inoculated with 50, 100, and 200mg/mL of TPPPS adjuvant recombinant pNZ-ompA/L. lactis, pure recombinant pNZ-ompA/L. lactis, blank-pNZ/L. lactis, and PBS, respectively, at 0–4, 10–14, and 24–28dpi. (A) At 29dpi, 20 mice were challenged by oral inoculation of LD50 of P. mirabilis. Faecal shedding was monitored daily by determining the CFU of P. mirabilis in samples. (B) Two weeks after the last inoculation, 20 mice from each group were challenged with oral inoculation of 10 LD50 of P. mirabilis. The survival status of mice was calculated with the following formula: Survival rate (%)=No. of surviving mice/Total No.×100.
Furthermore, we examined the protection of the recombinant pNZ-ompA/L. lactis on mice challenged with P. mirabilis. As shown in Fig. 6B, the protection rate in mice reached 100% in group pNZ-ompA-TPPPS (H), 85% in group pNZ-ompA-TPPPS (M), 75% in group pNZ-ompA-TPPPS (L), and 70% in pure pNZ-ompA (P<0.05). No mouse survived in groups blank-pNZ and Mock at four days post-challenge. Hence, the pure recombinant pNZ-ompA/L. lactis protects mice against P. mirabilis challenge limitedly. However, addition of 200mg/mL TPPPS to pNZ-ompA/L. lactis can completely protect mice against P. mirabilis infection.
DiscussionP. mirabilis is an enteric pathogenic bacteria that frequently causes animal infections and is thus considered as pathogenic diarrheagenic bacteria.21 Protective immunity against P. mirabilis mainly depends on specific mucosal immune responses induced by intestinal submucosal lymphoid tissues.22 For mucosal vaccination, LAB are widely used as live vaccine vehicles against various microbes because they present heterologous epitopes, thereby facilitating recognition by the immune system and mediating an immunoadjuvant effect with some of its components.23 In the present study, we constructed L. lactis harbouring the recombinant pNZ8149-ompA plasmid, which expressed exogenous P. mirabilis ompA protein based on a Nisin-controlled gene expression system. We also evaluated the immunogenicity of this recombinant pNZ-ompA/L. lactis in mice. TPPPS was first used as adjuvant to examine its immune enhancement effects on the oral vaccine. Our results demonstrated that oral immunisation with the recombinant pNZ-ompA/L. lactis can elicit both mucosal sIgA and systemic IgG immune responses. Furthermore, TPPPS adjuvant increased immunity levels induced by the recombinant pNZ-ompA/L. lactis.
Several expression systems are available for regulated and constitutive expression in L. lactis24; NICE system is the most often used. Nisin-regulated gene expression in L. lactis exhibits numerous characteristics: (1) overexpression of homologous and heterologous genes for functional studies to obtain large quantities of specific gene products; (2) metabolic engineering; (3) expression of prokaryotic and eukaryotic membrane proteins; and (4) protein secretion and anchoring in the cell envelope.11 This expression system is highly versatile and exhibits potential in pharmaceutical, medical, and food technology fields.25 In the past 10 years, some immune functional proteins expressed by the NICE system in L. lactis can be transported to the bacterial cell surface; consequently, both mucosal and systemic immune responses in the body conferred protection against pathogens.26
The recombinant pNZ-ompA/L. lactis can also significantly increase the production of IL-2, IFN-γ, and IL-4 in mice. IL-2, and IFN-γ, which belong to the Th1 cell cytokine family, play an important role in mediating cytotoxic effects and local inflammatory responses, assisting antibody generation, and participating in cellular immune responses.27 IL-4, as a Th2 cell cytokine, mainly promotes B cell proliferation and mediates humoral immune responses. However, we observed no significant differences on secretion of IL-10, which inhibits pro-inflammatory cytokine production and prevents macrophage apoptosis and tissue damage.28 This result demonstrated that a mixed Th1/Th2-based cell-mediated immune response was upregulated, and IL-10-mediated immunosuppression can be avoided through the common mucosal immune system during oral mouse immunisation with the recombinant pNZ-ompA/L. lactis.
Adjuvants are widely applied to enhance the immunogenicity of oral vaccines.29 Taishan P. massoniana pollens have been used as traditional medicine for thousands of years in China and are considered effective adjuvants for improving the immune system and facilitating immune responses in our laboratory.13,14 TPPPS contains three kinds of polysaccharides (named TPPPS1–3), and each component is composed of different monosaccharides and shows different dominant activities in anti-oxidant, anti-virus, and immunomodulation; hence, TPPPS exhibit synergistic effects on facilitating the immune function of organisms.30 As such, the use of TPPPS as adjuvant presented satisfactory effects on improving the immune responses of the recombinant pNZ-ompA/L. lactis oral vaccine by enhancing both mucosal and systemic immunity.
In conclusion, we demonstrated that orally-administered recombinant pNZ-ompA/L. lactis survives the transit of the upper gastrointestinal tract as well as expresses and secretes heterologous proteins, which induced specific mucosal and system immune responses against P. mirabilis. Moreover, TPPPS adjuvant presented good immune-enhancing effects on orally administered pNZ-ompA/L. lactis. This study presents the potential of the recombinant pNZ-ompA/L. lactis combined with TPPPS adjuvant on preventing P. mirabilis infection.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestNone of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
This project was funded by the National Key R&D Program of China (2017YFD0500706), Modern Agricultural Industry Technology System Foundation of Shandong Province (SDAIT-10-06), and the Chinese Medicine Antiviral Collaborative Innovation Center of Shandong Province Colleges (XTCX2014B0108).