A key point for maintenance of the immune system homeostasis is the balance between the capacity to recognize and fight exogenous molecules and the capacity to avoid auto reactivity. The disruption of this balance induces the progression of several immune diseases such as autoimmune diseases, allergies, infections or cancer.
A promising therapeutic approach to treat these diseases is immunotherapy. In cancer, both active and passive immunotherapies have been tested with promising results, such as the blocking of immunological checkpoints like CTLA-4 and PD-1. These treatments, in the market since a few years ago, aim to redirect the patient's immunological response by inhibiting the induction of regulatory T cells, both in the priming and effector phases.
This strategy sheds light on the immunological mechanisms that control the regulatory response mediated by T cells and opens new lines of research into other immunological diseases such as allergy, in which the induction of a regulatory response is necessary to avoid allergic progression and which is the main objective of allergen-specific immunotherapies available today.
The main feature of the immune system is its capacity to discriminate “self” and “non-self”. The key point for this feature is the interaction between the TCR molecules located on the surface of T cells and the complex formed by MHC:Antigen (Ag) located on the surface of antigen-presenting cells (APC). It is during this process of antigen presentation that the T cells need to discriminate between self and non-self antigens.1
This capacity is due to the wide TCR repertoire displayed by T cells. This repertoire is obtained during T cell development in the thymus. Here, T cell progenitors are subjected to sequential selection processes. First, they undergo a positive selection, where cells expressing functional TCR receptors with a high avidity for self-antigens are selected. Next, the selected cells experience a negative selection in which those with a high avidity for self-antigens are depleted and those with low- or intermediate-avidity are released into the peripheral blood. This process is known as central tolerance.2
Thus, once in circulation and in order to avoid an autoimmune response, when these cells that can recognize self-antigens with low or intermediate avidity meet cells expressing self antigens they display mechanisms like induction of anergy, apoptosis or immunosuppression, which constitutes the peripheral tolerance.2,3
Importantly, these processes are controlled by what is known as “immunological checkpoints” that we discuss later in this review.
Immune response against tumorsUnder homeostatic conditions, the immune system must be able to recognize exogenous stimuli and fight against them without affecting self-tissue. Dysregulation in this balance results in several pathologies either by excessive immune reactivity, which leads to autoimmune diseases or allergy, or by excessive tolerance, favoring tumor progression and infections.
In the case of cancer, tumor cells that were originally self-cells are identified by the immune system as non-self due to the expression of tumor-associated or tumor-related antigens.4 Recognition of these molecules by the immune system triggers the activation of effector CD8 T cells, NK cells, M1-macrophages that will together eliminate the tumor cells.5 However, this does not always occur. In some cases, the continuous interaction of the immune cells with the tumor cells induces a so-called “immunoedition”, which favors evasion of the immune system by the tumor.6,7 Usually, these results from the tumor cells’ capacity to reduce the expression of tumoral antigens, reduce the expression of MHC on the cell surface, or induce the secretion of immunosuppressive mediators such as TGFb or IL10. Together, all these “immunoeditions” facilitate a loss of immunogenicity by the tumor cells, the induction of immunosuppression and support tumor progression.8
Owing to the important role of the immune system in the progression and outcome of the tumor, since several years ago different strategies aiming to redirect or activate/inhibit the immune response in cancer have been tested as potential therapeutic tools. A systematic review of all the immunotherapeutic approaches in cancer will be presented, placing special emphasis on the most promising and novel strategies with a proven significant efficacy in several cancers. We, also, discuss how these strategies in cancer can contribute to improving several immunotherapeutic strategies developed for the treatment of other immune diseases such as allergy.
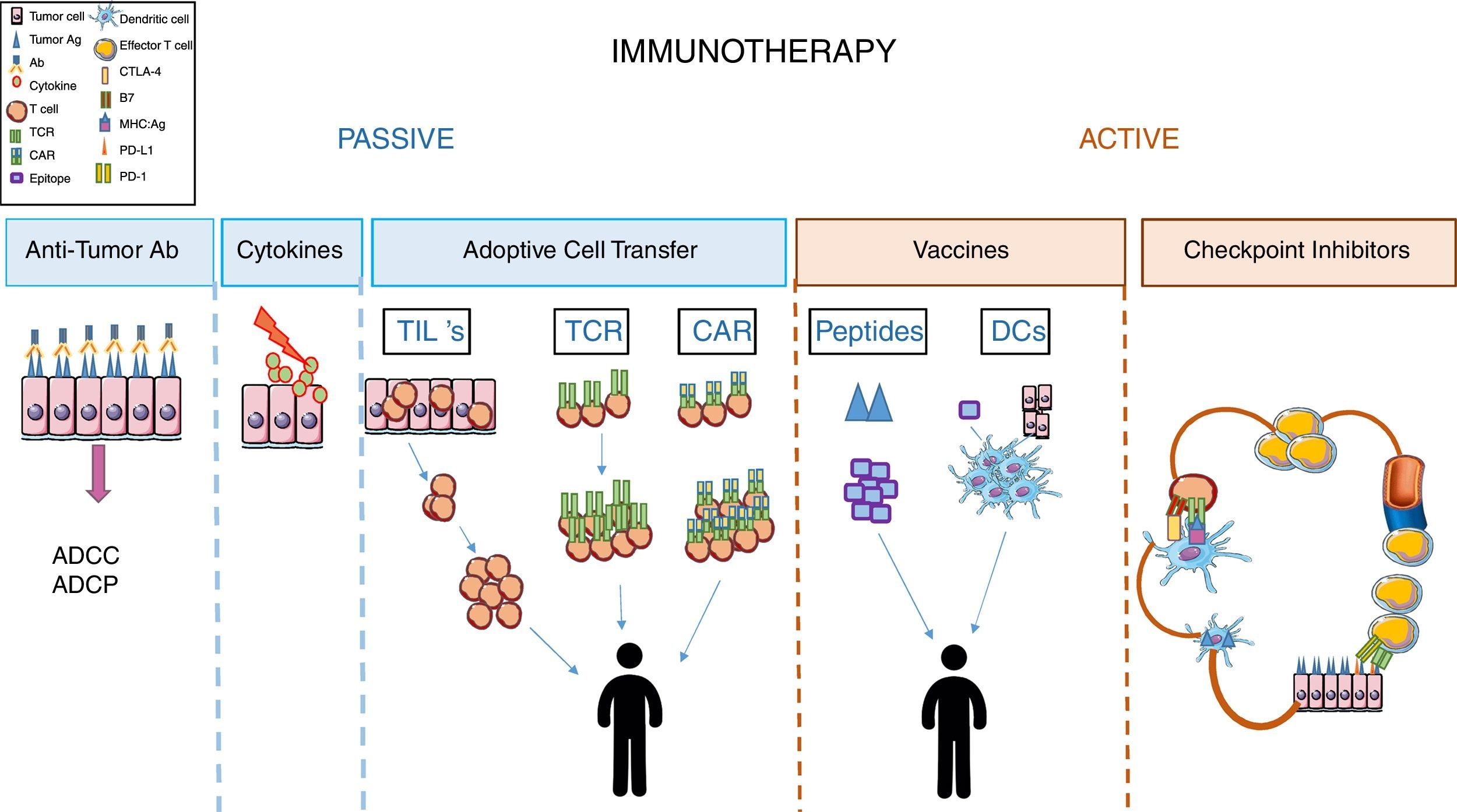
Immunotherapy in cancerImmunotherapy is considered as the treatment that aims to restore or intensify patients’ immune response (definition from the National Cancer Institute (NCI), NIH). In cancer, immunotherapy is divided into two groups depending on the therapeutic agent used and the condition of the patients’ immune system. In general, passive immunotherapy is used when patient immune response is weak or unable to respond, and involves using molecules or cells that, once in the patient's body, are able to compensate for the patient's immunological deficiency. On the other hand, active immunotherapy aims to stimulate the effector functions of the recipient immune system. In order to do this, the patient's immune system must be functional and responsive to the stimuli received9 (Fig. 1).
Passive immunotherapyAmong passive immunotherapy approaches, the use of tumor-specific monoclonal antibodies has been the most popular treatment in cancer for years. Tumor-targeting mAbs are the best-characterized form of anticancer immunotherapy and, perhaps, the most widely employed in the clinic. This treatment aims to specifically alter the signaling functions of receptors expressed on the surface of malignant cells, neutralize malignant cell signals such as VEGF-mediated angiogenesis, and selectively recognize cancer cells based on the expression of “tumor-associated antigen” and trigger cell death by antibody-dependent cell-mediated cytotoxicity (ADCC), or antibody-dependent cell-mediated phagocytosis (ADCP) of the target cell.9–11
Another therapeutic approach that was used several years ago, but much less nowadays, corresponds to treatment with cytokines such as IL-2.12,13
Adoptive cell transfer strategies in passive immunotherapy have been very promising in several types of cancer, although most studies were performed in melanoma.
A role for tumor infiltrated lymphocytes (TILs) in tumor progression has already been established.14 Their therapeutic value is based on their ability to recognize tumor antigens and induce a cytotoxic response to eliminate the tumor cells. The difficulty begins with the technical approaches. Thus, TILs need to be isolated from tumors after their resection, expanded in vitro, then injected into irradiated patients with administration of an IL-2 supplement.15–17 These procedures are not always possible, the number of TILs isolated is always a limiting factor, and the specific techniques required make it difficult to standardize this immunotherapeutic protocol.
Another possible strategy to re-direct the specificity of T cells is through genetic modifications of the TCRs so that they recognize specific tumor antigens.17,18 These engineered T cells can recognize tumor antigen in the context of MHC. A main benefit of this approach is that these cells can be generated from PBLs (not like TILs) and will be able to recognize both intracellular and extracellular antigens (not like CAR T cells). However, there are also significant disadvantages with this system, since the antigens recognized several times by these cells are, also, present in healthy tissue resulting in cytotoxicity and tissue damage. This is the case of MART-1 which is expressed in melanoma, but also in skin, retina and inner ear, since it is a marker of melanocyte differentiation.19 Another significant drawback to this immunotherapeutic approach is its MHC-dependence, as it is worthless in tumors cells that avoid immune surveillance by reducing the expression of MHC on their surfaces.20
In order to overcome these limitations in the strategy of “redirecting” T cell specificity, the next strategy developed was generation of CAR T cells. These cells result from the fusion of an scFV antibody with an intracellular signaling domain of the TCR complex. Recently, the fourth generation of CAR T cells was obtained by including in their design an intracellular domain of a costimulatory molecule that provides the “second signal” needed for T cell activations, and a domain for a cytokine to sustain T cell effector function.17,21
CAR T cells can recognize tumor antigen without MHC restriction (unlike engineered TCR T cells). However, they will only be used to recognize surface antigens, even when the antigens are expressed in healthy tissue (as occurs with engineered TCR T cells).20
The efficacy of this type of immunotherapy has mainly been tested in hematological tumors such as lymphomas, using antigen CD19 as the target.22
Active immunotherapy: blockage of immunological checkpointsRegarding active immunotherapy, the design of effective anti-tumoral vaccines has been a hot topic in the last decade.
To achieve this, several experimental approaches, using tumor peptide, DC vaccines or whole tumor cell lysates, such as immunogens, have been followed. The difficulty in performing the proposed experimental approaches, the low immunogenicity of several of the peptides used, the heterogenicity of tumor cells, in addition to the difficulty of obtaining them have resulted in this type of immunotherapy being a poor strategy to treat the majority of cancers.9,23,24
Finally, we come to the most promising treatment today used in clinical practice, which constitutes a great advance in the treatment of several cancers. This strategy of active immunotherapy uses monoclonal antibodies as inhibitors of “immunological checkpoints”, which control the initial and effector phases of T cells in their response to tumor antigens.
The immunological cycle in cancer begins with the recognition of tumor antigens by APC. Once activated, the APCs migrate into the lymph nodes and present tumor antigens to naïve T cells. In order to induce the activation and proliferation of T cells, in addition to the interaction of TCR with the MCH:Ag complex, the presentation must include signaling mediated by “co-stimulatory molecules”. In the absence of this second signal, T cells differentiate into non-responder cells (regulatory cells or anergic cells). So, regulation of this first interaction is considered to be an important immunological checkpoint to target during immunotherapy, in order to “redirect” the T cell response. Later on, effector T cells migrate into the blood vessels and extravasate into the tumor to perform their effector functions, proceeding to kill the cancer cells. Since, during the effector phase, several mechanisms prevent the killing of the tumor cells: by activated T cells, induction of regulatory mechanisms, such as the release of immunosuppression mediator at the tumor site, or induction of costimulatory molecules like PDL-1 that trigger a regulatory response in the T cells at the effector site, this step in the cancer cell cycle constitutes another essential checkpoint to target during cancer immunotherapy.25–27
Two signals are required to achieve complete and functional T cell activation. The first signal is mediated by the interaction of TCR molecules from naïve T cells with the MHC:Ag complex presented on the surface of the APC. The second signal is mediated by the costimulatory molecules expressed on the APC (like the B7 family, among others) and their ligands (CD28/CTLA4/PD1) expressed on the T cell surface.
There are several families of costimulatory molecules and their ligands on the T cells that favor either activation or inhibition of their effector function. This directs the fate of T cell activation. Thus, absence of the second signal will drive T cell anergy or apoptosis, whether the interaction of costimulatory molecules with activating ligands will induce T cell activation and proliferation and the interaction with inhibitory ligands will induce T cell arrest and tolerance induction. This explains the importance of the second signal mediated by the costimulatory molecules as immunological checkpoints.28–30
An essential concept to take into account during cancer immunotherapy is that tumor cells are able to evade an immune response by inducing peripheral tolerance. Therefore, the checkpoints that regulate tolerance induction are considered to be essential therapeutic targets.
Here, we focus especially on checkpoint control by the costimulatory molecules CTLA4 and PD-1, since they have already been approved for use in cancer immunotherapy with promising results.30
CTLA4 is considered an inhibitory molecule since it can prevent naïve T cell activation of potential autoreactive T cells. This process usually takes place in the lymph nodes during the activation phase. PD-1 regulates previously activated T cells during the effector phase, preferentially at the tumor site.31
Both molecules have the same negative effect on T cell activation. However, they differ in the time and place where they induce the inhibitory effect. CTLA-4 is mainly expressed on T cells; its ligands (B7) are mainly expressed on APCs, and can inhibit naïve T cell activation in the priming phase. On the other hand, in addition to PD-1 being expressed on T cells, it is also expressed on B cells, myeloid cells and non-hematopoietic cells. Its ligands (PDL-1 and PDL-2) are, also, widely distributed in hematopoietic and non-hematopoietic cells, and on tumor cells because of the inflammatory environment.32,33
CTLA-4 is stored in intracellular vesicles in the naïve T cells located in the lymph nodes. When the APCs present the MHC:Ag complex, if the interaction with the TCR is weak, T cells will express on their surface CD28, an activation ligand for B7 costimulatory molecules, and the resulting response will be T cell proliferation, IL2 production and increased survival. However, if the TCR:MHC:Ag complex interaction is strong, CTLA-4 will be transported to the T cell membrane and the co-stimulatory molecules B7 will bind its inhibitory ligand, CTLA-4, due to a higher affinity than for CD28. These interactions will induce a net inhibitory signal with reduced IL2 production, cell survival and proliferation, which will favor peripheral tolerance.34 On the other hand, PD-1 mainly exerts its role at the tumor site. Once the effector T cells recognize the tumor antigens, expression of the costimulatory molecule PD-1 increases on the surface. At the same time, tumor cells, due to the inflammatory surrounding environment, express PD-1 ligands (PDL-1 and PDL-2) and the interaction of both molecules at the effector site will induce a negative signal in the T cells and a tolerogenic profile, allowing tumor cell evasion and cancer progression.31,35,36
Monoclonal antibodies targeting CTLA-4 were approved for cancer immunotherapy in 2011. Although the clinical outcome was very promising, the toxicity rate of the treatment was a serious concern. More recently, in 2014, the FDA approved anti-PD-1 treatment with even more significant results, mainly in melanoma and lung cancer. Blockage of PD-1 reduces the magnitude of T cell activity at the effector site. This reduces its activity spectrum compared to the effect of anti-CTLA4 blockage. This might explain less adverse effects in anti-PD1 treatment.20,30
More recently, Chinese researchers began the first attempt to treat metastatic non-small-cell lung cancer by immunoedition using the revolutionary CRISPR–Cas9 technique. They have disabled the gene code for PD-1, aiming “redirecting” T cell activation. Dr. Lu's team plan to culture the edited cells, increase their number, and inject them back into the patient with metastatic non-small-cell lung cancer, in the hope that, without PD-1, the edited cells will attack and defeat the cancer.
Lessons from cancer immunotherapy in allergy immunotherapyThe role of costimulatory molecules in allergy and asthma could provide new insight into the design of therapeutic approaches. It is well established that the deleterious allergic response is initiated by T-cell recognition of MHC:Ag complexes at the surface of APCs. While this first signal gives antigen specificity to the adaptive immune response, a second nonspecific costimulatory signal is required by T cells to become fully activated. Depending on the type of molecules involved, this secondary signal can promote the development of an inflammatory allergic reaction or favor immune regulation.
Thus, CTLA-4, which is present on the regulatory T-cells (Treg), is found to be involved in the induction of oral tolerance and suppression of food allergy, demonstrating the relevance of the “second signal” in the development of food allergy. Recently, the role of CTLA-4 in suppressing sensitization or inducing oral tolerance has been reviewed37 and has opened up a new dimension in food allergy research due to its great impact on T-cell activity suppression.38 The genomic regions of CTLA-4 genes including chromosome 2q33 have been found to be associated with the development of allergy. CTLA-4 gene polymorphisms are responsible for determining the Th1 and Th2 balance.39
To date, avoidance of the food causing the allergic manifestation in susceptible individuals is the most common recommendation by clinicians. Some molecules, like anti IgE, IL-4 and regulatory cells (Treg), have been considered as potential tools to treat food allergies, but these studies are still in experimental stages. The role for CTLA4 in the generation of oral tolerance opens up the possibility of improving current immunotherapeutic approaches.
Additionally, using murine models a role for the costimulatory molecules PD-1 has been described in allergic asthma. Likewise, PD-1 with its ligands, PD-L1 and PD-L2, was shown to regulate T-cell activation and tolerance. Hence, upon recognition and activation, pulmonary dendritic cells express PD-L1 and PD-L2. This report demonstrates that PD-1/PD-L1 interaction produces a Th2 response with more IL-4 production, leading to increased airway hyperreactivity, whereas PD-1/PD-L2 interaction initiates a Th1-type response with increased expression of IFN-γ, subsequently, reducing airway hyperreactivity. Simultaneous expression of PD-L1 and PD-L2 neutralizes the single effects and does not lead to immediate polarization of T cells.40
In addition to using immunological checkpoint control to improve allergy immunotherapy, novel strategies using DC-based vaccines have, also, been described. Sirvent et al., demonstrated that allergoids coupled to mannan through a method that avoids sugar oxidation might well represent a novel hypoallergenic vaccine, which enhances allergen uptake and promotes the generation of functional FOXP3 Treg cells through PD-L1 in human subjects.41
In summary, generations of knowledge regarding regulation of the immune response have been fruitful in developing novel immunotherapies in cancer. Those strategies that prove to be successful should be taken into account in the design of future immunotherapy strategies in other diseases such as allergy.
Conflict of interestDr. Escribese declare no conflict of interest.
The Dr. Barber declare having acted as Scientific Advisor in the last 12 months for the following Companies: ALK-Abello and AIMMUNE.
This work was supported by ISCIII (Project number PI15/02256 and PI13/00477), and by Fundación Mutua Madrileña (AP158912015).