Probiotics could be beneficial to health and some of them have shown to modulate immune responses.
AimThe aim of this study is to investigate if the probiotic strains including Lactobacillus and Pediococcus strains are able to alleviate allergic reactions in an ovalbumin-induced airway allergy model.
MethodsLactobacillus multi-species preparation (LMP) was gavaged to BALB/c for total six weeks and BALB/c was challenged with ovalbumin in the last two weeks. A barometric whole-body plethysmography was used to assess enhanced pause (Penh) of airway hyperreactivity (AHR). Immunoglobulins (Ig) such as IgE, IgG1, IgG2a and cytokines such as IL-12, IFN-γ, IL-4, IL-5, TNF-α and IL-13 in bronchoalveolar lavage fluid were assayed using ELISA kits.
ResultsThe results showed this LMP significantly reduced Th2 cytokines and enhanced Th1 cytokines production. OVA-specific IgE and IgG1 was lower in the probiotics-treated mice whereas IgG2a was increased. Most importantly, this murine model showed LMP supplementation significantly reduced AHR.
ConclusionsOverall, this Lactobacillus multi-species preparation seemed to suppress OVA-sensitized airway hyperreactivity, thus serving as a possible candidate for therapeutic uses for allergic airway symptoms.
Asthma is a severe health issue worldwide. One of the etiologic agents for asthma is the exposure to allergens, which cause excess mucus secretion, airway obstruction, inflammation and airway hyperreactivity (AHR).1 The pathogenesis of asthma is largely mediated by Th2 responses, which consist of a complex network of cytokines interaction. The Th-2 type cytokines such as interleukin (IL)-4, -5 and -13 are involved in pathogenesis of allergic response and eosinophil infiltration in AHR.2–4 Antigen presenting cells (APCs) such as dendritic cells recognize allergens such as dusts, viral particles and then activate Th2 response. The activated Th2 cells facilitate production of IgE, IgG1 by B cells, degranulation of mast cells and eosinophilia via cytokine secretion. The ovalbumin-induced acute inflammation on airway mucosa gradually goes to a chronic inflammation and leads to airway remodeling. Thus, dampening excess Th2 responses in asthmatic subjects is considered as a plausible approach in ameliorating AHR. Th1 responses on the other hand are important in regulating Th2 responses. APCs such as dendritic cells secret IL-12 to drive Th1 cell differentiation, which induce IFN-γ and IgG2a production. IFN-γ has both anti-asthmatic and pro-inflammatory effects. It tends to inhibit the proliferation of Th2 cells, stimulate preferential expansion of Th1 cells and promotes macrophage killing in response to intracellular microbes and parasites. TNF-α also has the potential to stimulate induction of cytokine, therefore it could play an important role in the pathogenesis of airway remodeling. As a conclusion from the above, enhancing Th1 responses seems to be effective in counter-acting excess Th2 responses in alleviating AHR.
Probiotics refer as microorganisms that confer health benefits to the host.5 It is known from mouse studies that intestinal microflora are necessary to shape our immune system as germ-free mice do not have a well-functioning immune system.6 The surface molecules on the bacteria are recognized by APCs and stimulate T cell development. Bifidobacterium and Lactobacillus probiotics have been shown to alleviate various allergic conditions. For example, B. bifidum BGN4 has been shown to alleviate food allergy by inhibiting IgE and IgG production.7Lactobacillus spp. have been widely studied in food and airway allergy models.8–11Lactobacillus rhamnosus GG was shown in lowering Th2 cytokines and IgE production in an ovalbumin-induced air inflammation mouse model.11
Another example, Lactobacillus casei shiota cannot only lower the production of Th2 cytokines such as IL-4, IL-5, but also increase production of Th1-associated cytokines including IFN-γ and IL-12 in a food allergy model.12 Overall, the restoration of Th1 and Th2 balance seems the most sensible approach to alleviate allergic conditions.
In this study, the potential probiotic preparation contains two Lactobacillus plantarum strains LP109 and LP110 and Pediococcus acidilactici PA320. We screened several probiotic strains which demonstrated the potential to modulate AHR and immunoglobin production in a mouse model (data not shown) and chose the potential ones for this experiment. A previous study by Nawaz demonstrated a multi-species preparation including Bifidobacterium breve M-16V and L. plantarum NumRes8 reduced IL-4 and IL-5 production.13 To the best of our knowledge, no study has reported the anti-allergy effect of P. acidilactici in the airway allergy model before. The aim of this study is to investigate if the probiotic preparation including Lactobacillus and Pediococcus strains is able to alleviate allergic reactions in an ovalbumin-induced airway allergy model and whether the anti-allergy effect are exerted by either or both Th1 and Th2 responses.
Materials and methodsAnimalsBALB/c mice were purchased from BioLasco, Taiwan Co., Ltd. (Taipei, Taiwan). Six-week old female mice were first acclimatized in an animal room for a week at 20–23°C with 12-hour light/dark cycles. The animals were provided with Lab Diet® 5010 Rodent Diet (PMI Nutrition International, USA) and sterile reverse osmosis water ad libitum. The study was approved by the Institutional Animal Care and Use Committee (IACUC) in Hungkuang University (Taichung, Taiwan).
LMP preparationThe lactobacillus multi-species preparation (abbreviated as LMP) containing L. plantarum LP109, L. plantarum LP110 and P. acidilactici PA320 was fermented and freeze-dried by New Bellus Enterprises Co. Ltd. (Tainan, Taiwan), the powder (3.5×1011CFU/g) was stored at −20°C. Daily intake of LMP was 1.8g or 7.2g per day for adult. The animal equivalent dose using body surface area was 0.39g/kg BW (low dose) or 1.56g/kg BW (high dose).14 The probiotic powder was suspended in sterile phosphate-buffer saline (PBS) for gavage.
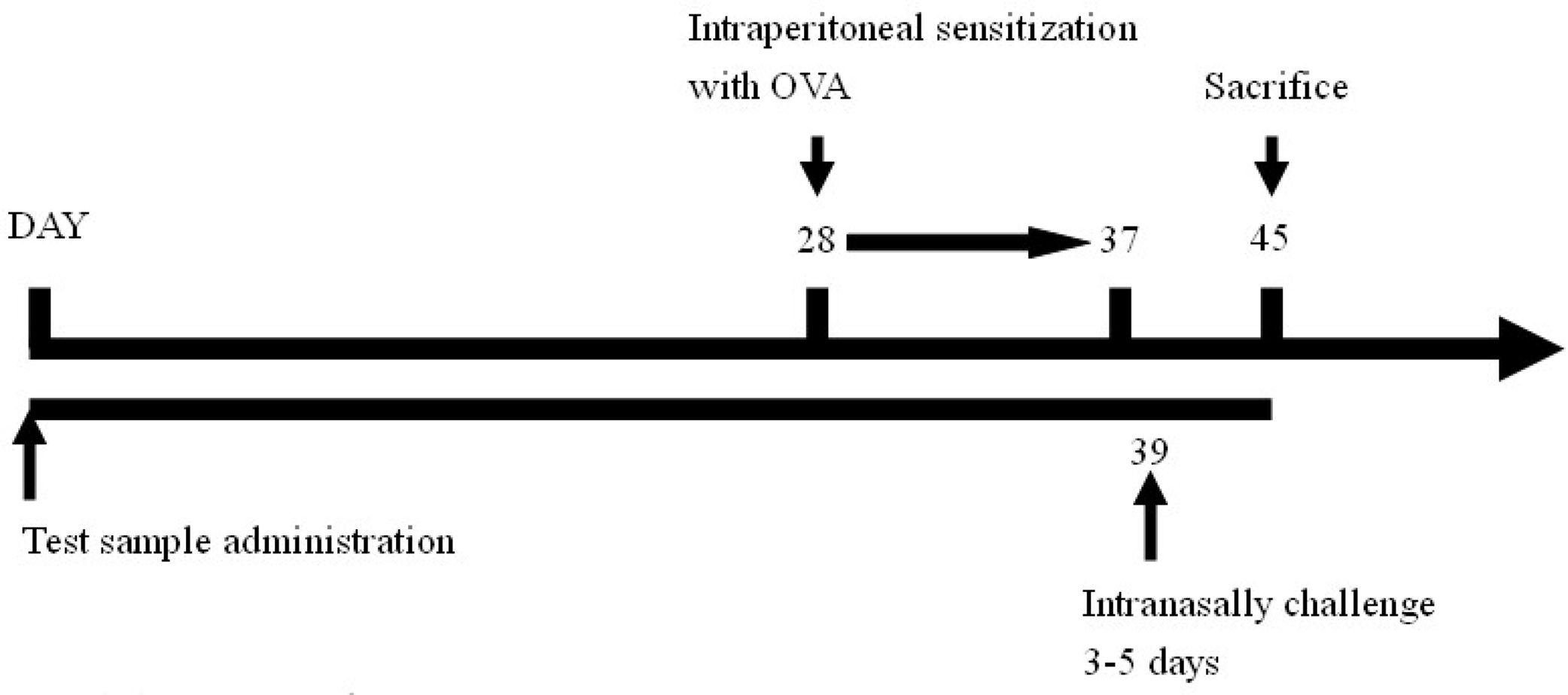
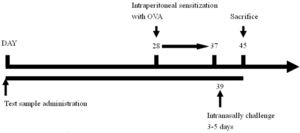
Experimental regimeForty animals were divided into four groups, include naïve group, OVA control group (OVA challenge, PBS gavage), lactobacillus multi-species preparation (abbreviated as LMP) low-dose group (L.L) (OVA challenge, LMP at 0.39g/kg BW) and LMP high-dose group (L.H) (OVA challenge, LMP at 1.56g/kg BW), with ten mice in each group. The LMP was orally gavaged to animals daily for the first four weeks.
After that, animals were intraperitoneal sensitized using OVA (0.5mg OVA mixed with 2mg Al(OH)3, 0.1ml/10g BW per mouse) for ten days, then intranasally challenged with OVA (2mg/ml, 75μl per mouse) for five days15; airway hyperreactivity was then assessed using barometric whole-body plethysmography. After animal sacrifice, bronchoalveolar lavage fluid was collected for further analysis.
The experimental timeline is as follows:
Assessment of airway hyperreactivity by barometric whole-body plethysmographyAt the end of the last intranasal challenge, the animals were placed in a chamber for the whole-body plethysmograph (Buxco, Troy, NY, USA) for 3min, and the pressure differences between the chamber and a reference chamber were measured using a differential pressure transducer connected to an amplifier for recording. The respiratory cycle of the mouse would cause the change of the box pressure and the signal. The enhanced pause (Penh) reflects changes in the wave form of the box pressure signal during both inspiration and expiration. Penh was calculated as (Te-Tr)/Tr (PEP/PIP), where Te is expiratory time (s); Tr is relaxation time (s), defined as the time of pressure decay to 30% of the total expiratory pressure signal (area under the box pressure signal at expiration); PEP is peak expiratory pressure (ml/s); and PIP is peak inspiratory pressure (ml/s). The Penh values were recorded.16
Harvest of bronchoalveolar lavage fluid and cell countThe trachea was cannulated after mouse was interperitoneally injected with pentobarbital (1.5g/kg bw) and the cannula was clamped. One ml of Hank's balanced salt solution (HBSS) was lavaged into the lung and retrieved from the lung after gentle message. The whole process was repeated three times. The retrieved bronchoalveolar lavage fluid (BALF) was centrifuged and stored frozen (−70°C) for further analysis. Cell preparations were stained with Liu's stain method and a differential cell count was performed on 300 cells or more. The cell proportions were determined by cell counting using a hemocytometer under an optical microscope.
Evaluation of immunoglobulins and cytokines in BALFThe OVA-specific antibodies IgE, IgG1 and IgG2a in BALF were assayed using the commercial ELISA kits (Bethyl Laboratories, Inc. Montgomery, TX, USA).17 Cytokines such as IL-4, IL-5, TNF-α, IL-13, IL-12 and IFN-γ were also assayed using the commercial ELISA kits (eBioscience, San Diego, CA, USA).18,19
Statistical analysisMeans and standard deviations were calculated for the collected data. The data were analyzed by one-way analysis of variance (one-way ANOVA), followed by post hoc Duncan's multiple range testing. A P<0.05 value was considered as the minimum level for significance.
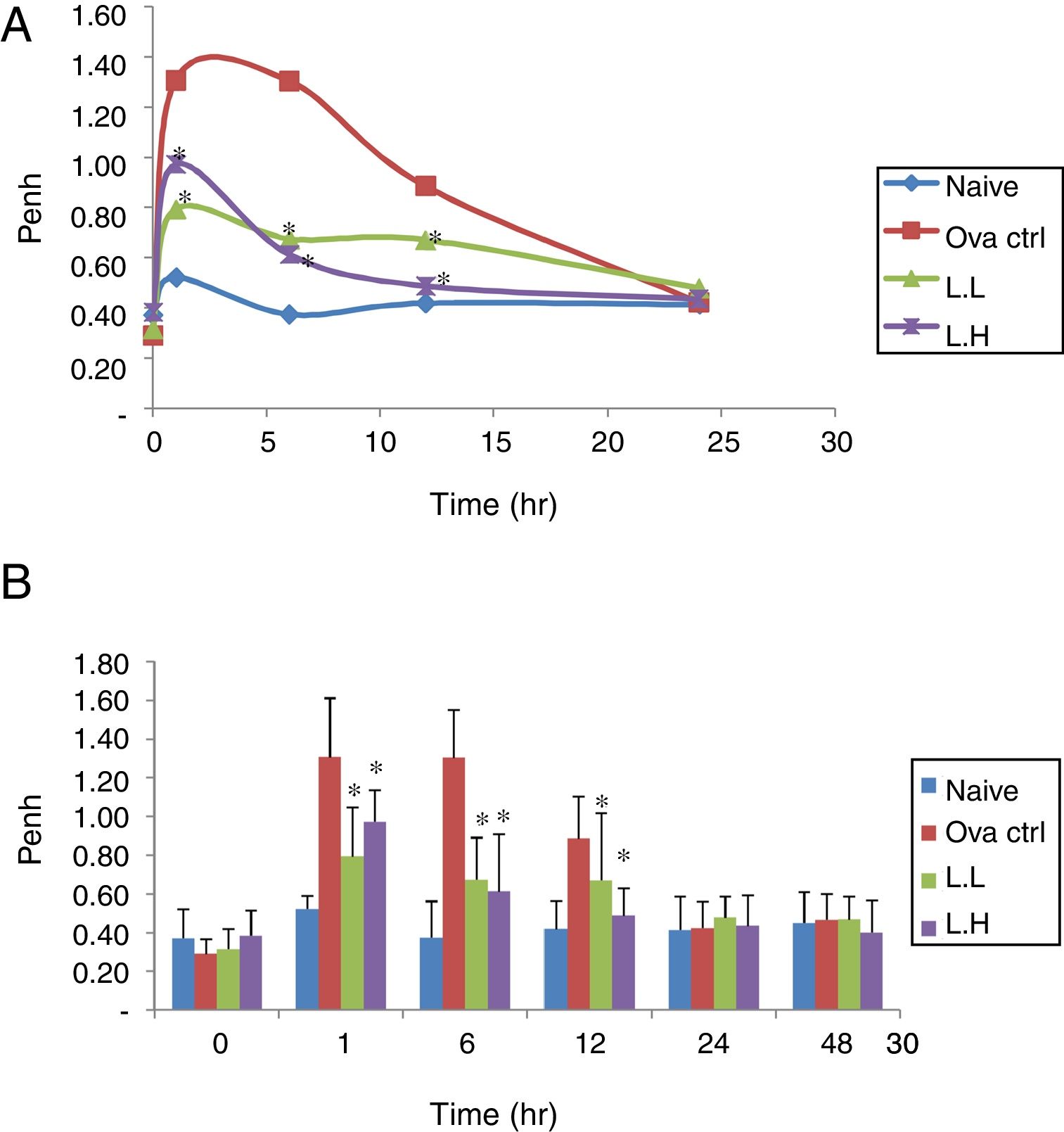
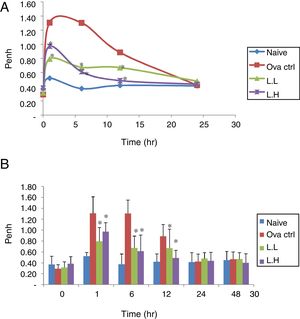
ResultsEffect of LMP administration on AHR in OVA-sensitized miceAHR was measured using a non-invasive whole-body plethysmography. The airway resistance was measured in animals after five days of intranasal OVA-challenge. The measurement was expressed as Penh (Enhanced Pause). The higher the Penh value the higher the airway resistance. In Fig. 1, Penh values for naïve animals over the time course showed minimal fluctuations. At 1, 6, 12h, the naïve group showed lower Penh values (P<0.05) compared with OVA control group (Fig. 1A and B). Low dose LMP and high dose LMP group showed lower Penh values at 1, 6, 12h (P<0.05) compared with the OVA control group (Fig. 1).
Effect of oral administration of LMP on the airway response measured at different time points after the last OVA challenge as expressed by Penh. The Penh value of mice was measured using a non-invasive whole-body plethysmography at 0, 1, 6, 12, 24 and 48h after the last OVA challenge. (A) Penh values were expressed as means, n=10. (B) Penh value, each value represents the mean±SD, n=10. Groups are normal control (naive), OVA-challenged control (OVA ctrl), low dose LMP (L.L) and high dose LMP (L.H). A difference between the LMP groups and OVA ctrl group at the same time point was considered statistically significant when P<0.05 (*).
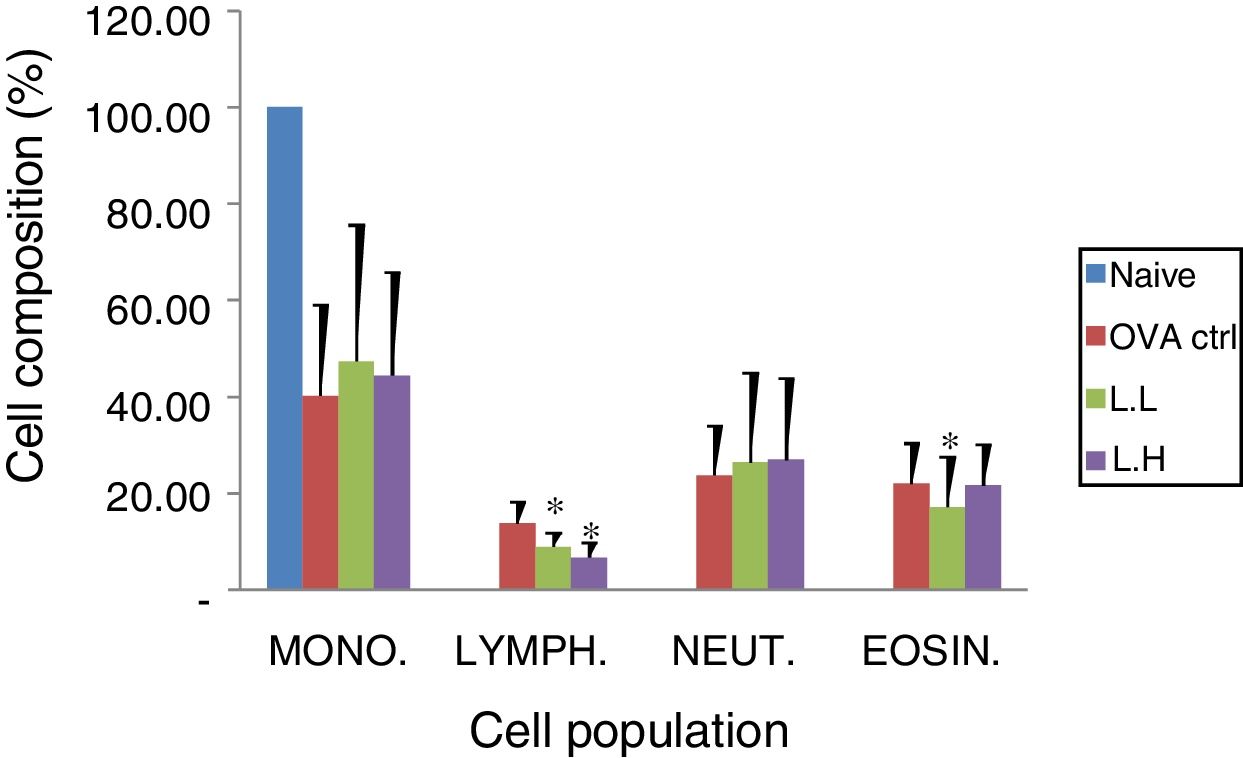
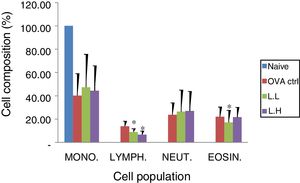
Animal showed normal body weight increase and food intake during the experiment period (data not shown). No significant difference (P>0.05) on numbers of monocytes and neutrophils were observed between the OVA control group and both
LMP dose groups (Fig. 2). A lower lymphocyte count (P<0.05) was found in both LMP dose groups compared with the OVA control group. The number of eosinophils was significantly lower (P<0.05) in the low dose LMP-treated group than the OVA control group (Fig. 2).
Effect of oral administration of LMP on lung tissue inflammatory cell infiltration in OVA-sensitized mice. Cell preparations were stained with Liu's stain method and a differential cell count was performed using a hemocytometer. Each value represents the mean±SD, n=7. Groups are normal control (naive), OVA-challenged control (OVA ctrl), low dose LMP (L.L) and high dose LMP (L.H). Mono: monocyte, LYMPH: lymphocyte, NEUT: neutrophil, EOSIN: eosinophil. A difference between the LMP groups and OVA ctrl group was considered statistically significant when P<0.05 (*).
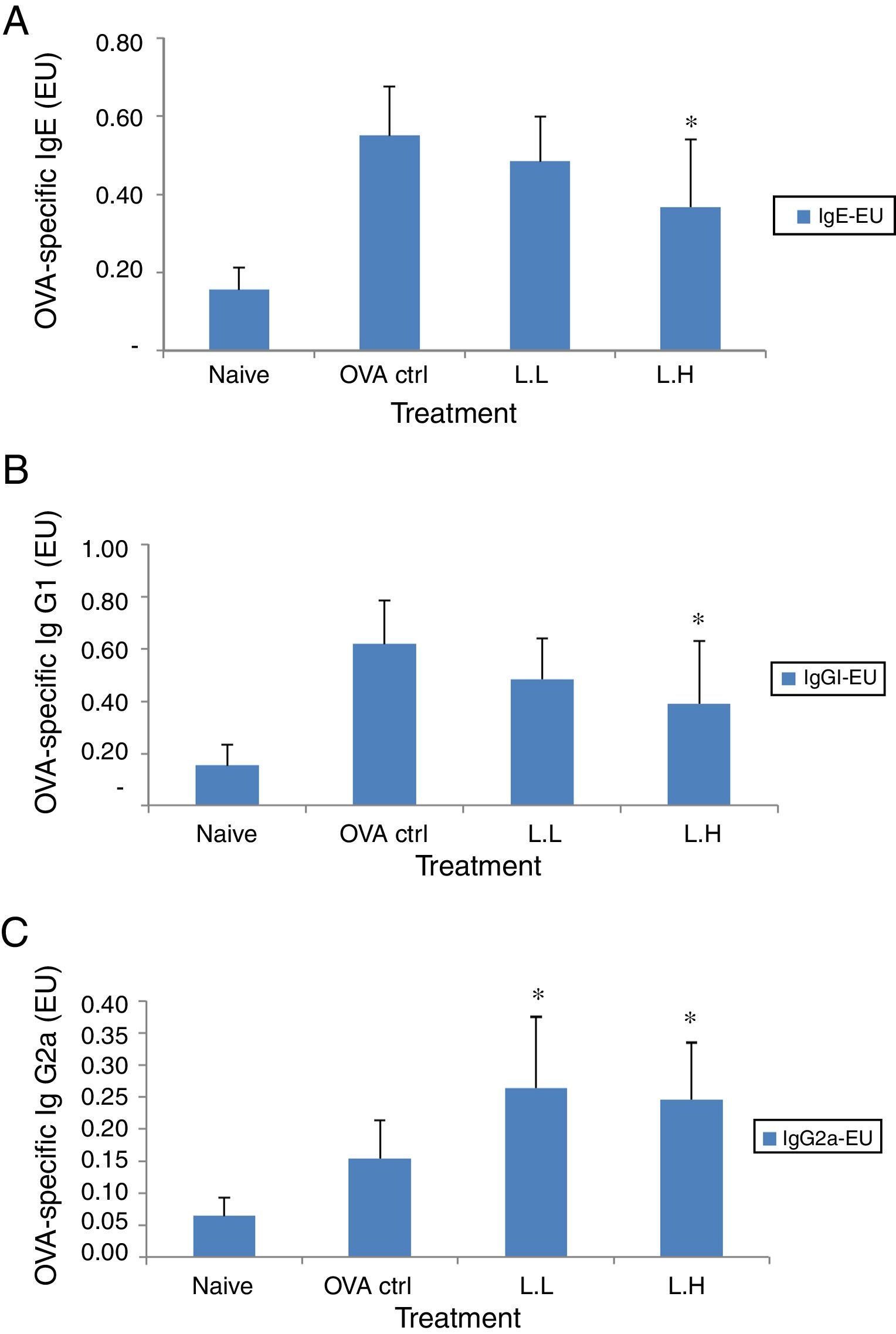
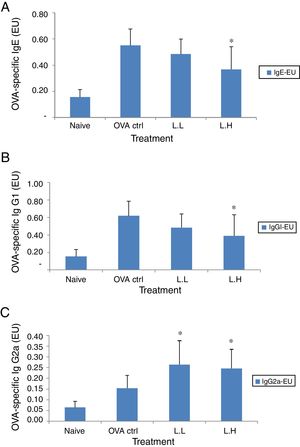
In BALF, the high dose LMP-treated group showed lower levels of OVA-specific IgE and IgG1 (P<0.05) compared with the OVA control group (Fig. 3A and B). The OVA-specific IgG2a level was significantly higher in both LMP dose groups (P<0.05) than the OVA control group (Fig. 3C).
Effect of oral administration of LMP on immunoglobulins in BALF of OVA-sensitized mice. (A) OVA-specific IgE (B) OVA-specific IgG1 and (C) OVA-specific IgG2a in BALF of mice. Each value represents the mean±SD, n=7. Groups are normal control (naive), OVA-challenged control (OVA ctrl), low dose LMP (L.L) and high dose LMP (L.H). A difference between the LMP groups and OVA ctrl group was considered statistically significant when P<0.05 (*).
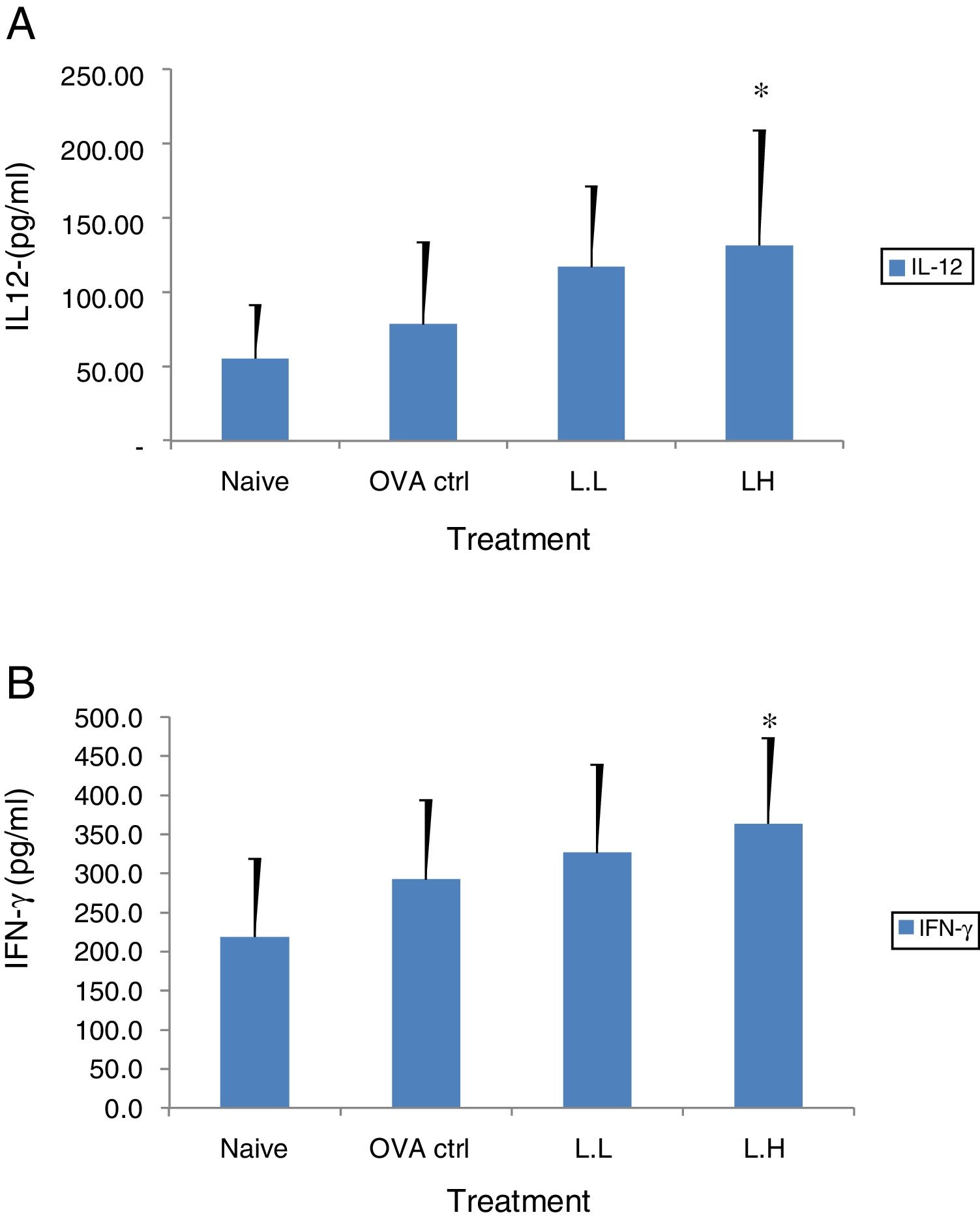
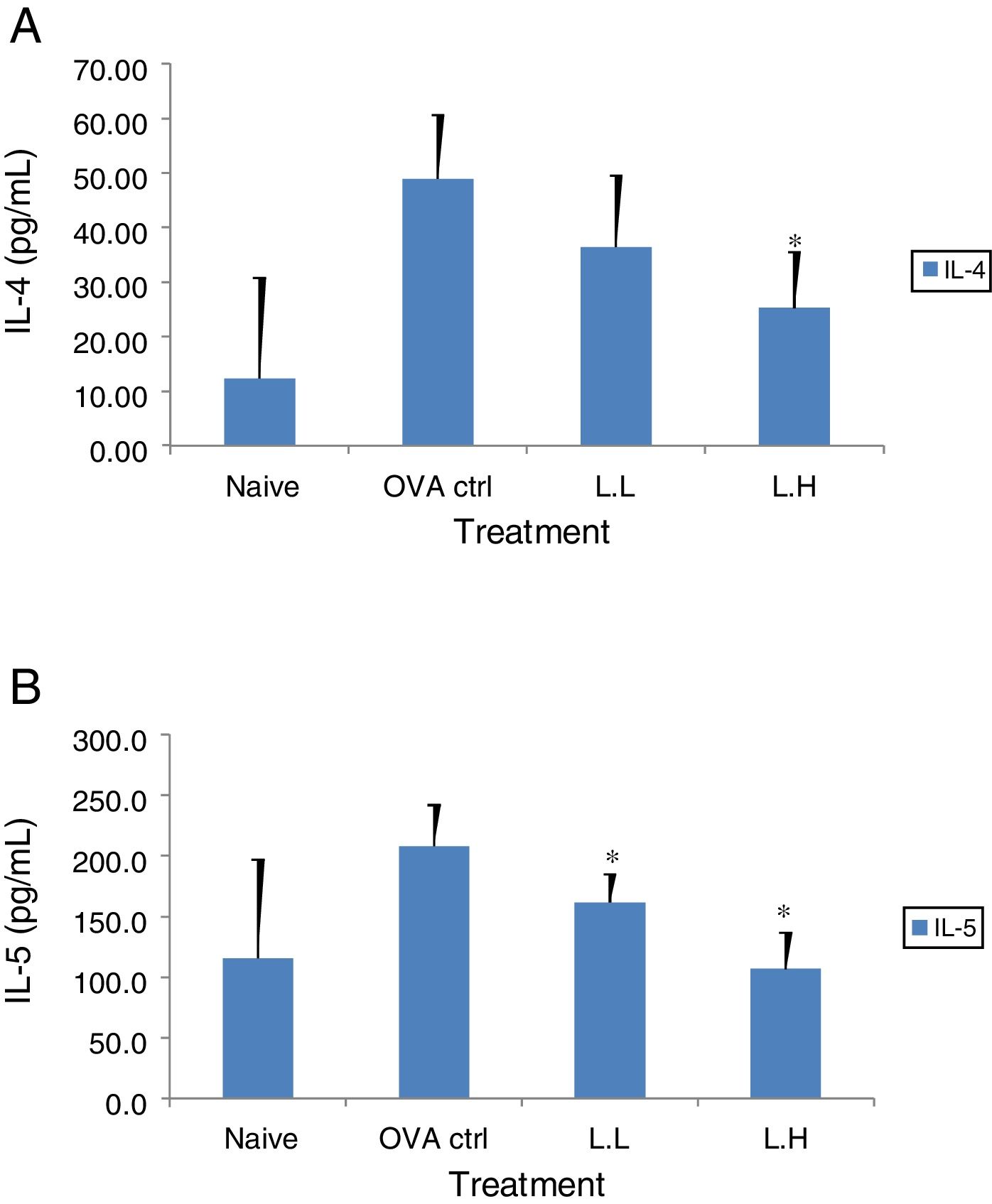
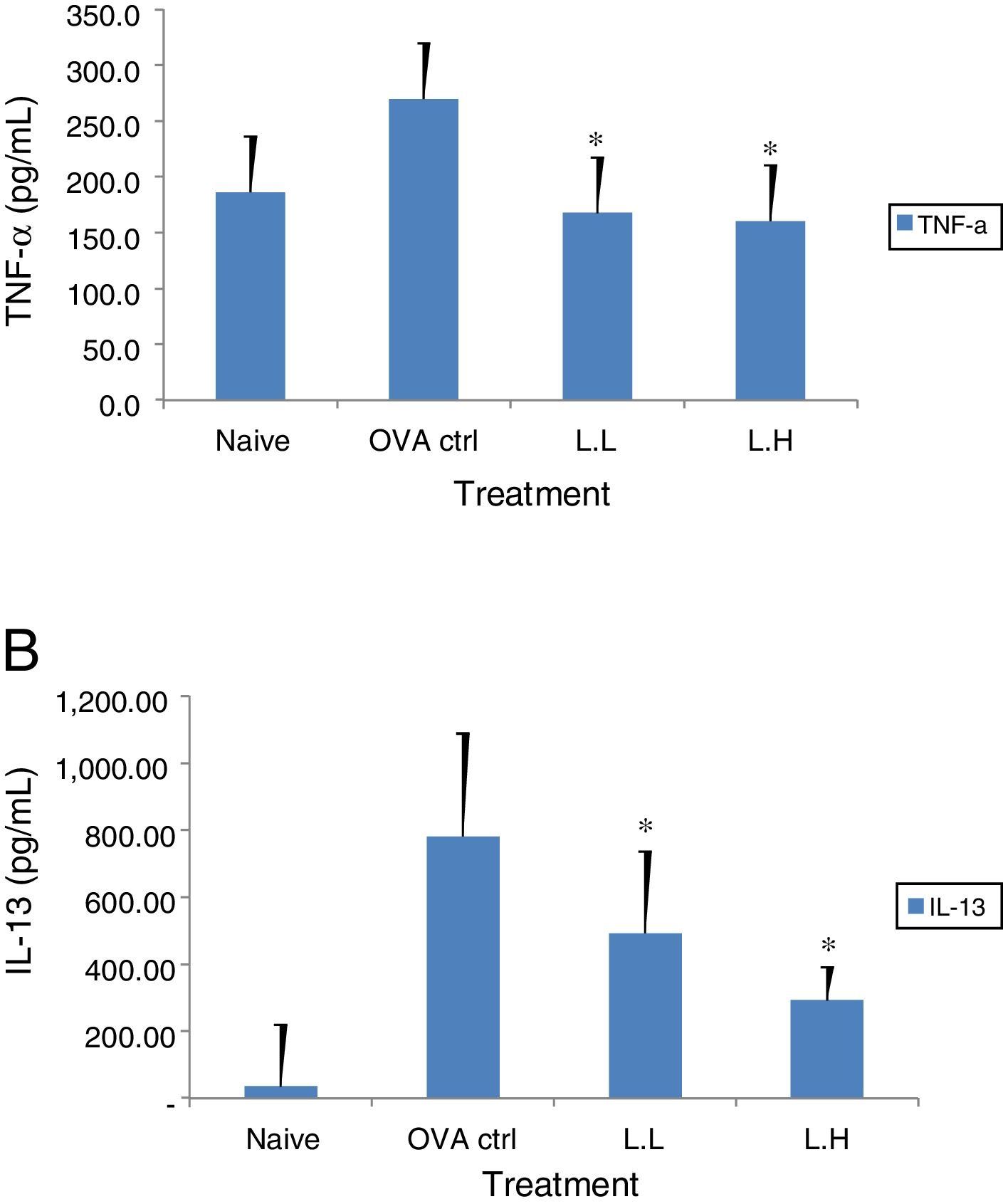
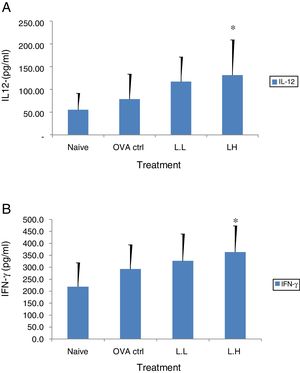
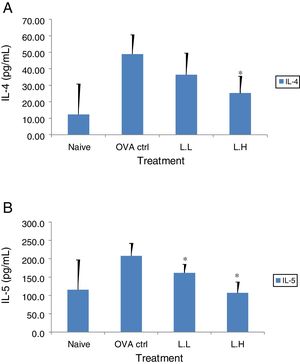
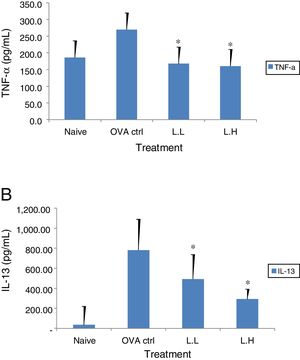
The high dose LMP-treated group showed higher IL-12 and IFN-γ secretion (P<0.05) compared with the OVA control group (Fig. 4A and B). While IL-4 was lower in the high dose LMP-treated group (P<0.05) and IL-5 was lower in both LMP dose groups (P<0.05) (Fig. 5A and B). The level of TNF-α and IL-13 in BALF of both LMP dose groups were lower than the OVA control group (P<0.05) (Fig. 6A and B).
Effect of oral administration of LMP on IL-12 and IFN-γ production in BALF of OVA-sensitized mice. The concentration of (A) IL-12 and (B) IFN-γ in BALF of mice were assayed using ELISA. Each value represents the mean±SD, n=7. Groups are normal control (naive), OVA- challenged control (OVA ctrl), low dose LMP (L.L) and high dose LMP (L.H). A difference between the LMP groups and OVA ctrl group was considered statistically significant when P<0.05 (*).
Effect of oral administration of LMP on IL-4 and IL-5 production in BALF of OVA-sensitized mice. The concentration of (A) IL-4 and (B) IL-5 in BALF of mice were assayed using ELISA. Each value represents the mean±SD, n=7. Groups are normal control (naive), OVA-challenged control (OVA ctrl), low dose LMP (L.L) and high dose LMP (L.H). A difference between the LMP groups and OVA ctrl group was considered statistically significant when P<0.05 (*).
Effect of oral administration of LMP on TNF-α and IL-13 production in BALF of OVA-sensitized mice. The concentration of (A) TNF-α and (B) IL-13 in BALF of mice were assayed using ELISA. Each value represents the mean±SD, n=7. Groups are normal control (naive), OVA-challenged control (OVA ctrl), low dose LMP (L.L) and high dose LMP (L.H). A difference between the LMP groups and OVA ctrl group was considered statistically significant when P<0.05 (*).
The BALB/c strain has been commonly used for allergy model.20 Upon OVA sensitization, these animals displayed airway hyperreactivity, increased IgE levels and elevated numbers of eosinophils in the BALF. Th2 cytokines such as IL-4, IL-5, IL-13 are known for promoting allergic responses including eosinophil infiltration, airway hyperreactivity, IgE production and airway remodeling. All of these features resemble that in humans.21 It is clear that the asthmatic allergy is a pathogenic Th2-driven response.
After all six weeks of probiotic feeding, both doses of LMP showed a similar trend on alleviating airway hyperreactivity and regulating productions of OVA-specific antibodies and cytokines in this OVA-sensitized mouse model. Considering all the data, it seemed that both doses were promising on alleviating AHR in this model. The Penh value was reduced in both LMP dose groups. The level of OVA-specific IgE and IgG1 was reduced in high does LMP-treated group, whereas IgG2a level was enhanced in both does LMP dose groups. The above results indicated LMP suppressed allergic reactions related to airway hyperreactivity, production of OVA-specific IgE, IgG1 and IgG2a. Th1 associated cytokines IL-12 and IFN-γ were enhanced in high dose LMP-treated group, while Th2 associated cytokines IL-4, IL-5, IL-13 were reduced in both dose groups. The level of TNF-α were reduced in both dose groups. The data suggested LMP may drive T cell toward Th1 pathway and counter-acting excess Th2 responses. The eosinophils in the low dose LMP-treated group were reduced but this was not observed in the high dose LMP group. It may be due to the anti-asthmatic and pro-inflammatory effects of IFN-γ in the high dose LMP-treated group. The presence of IFN-γ may modulate the effects of IL-13 in the lungs.22 In Ford's mouse model,22 the blockade of IL-13 partially inhibited airway hyperreactivity and goblet cell hyperplasia but not inflammation. The IFN-γ in high dose LMP-treated group may play double-sides effects (inhibiting some, potentiating others) on IL-13-induced changes in the lung. However, this speculation may need further investigation.
In summary, we demonstrated that a lactobacillus multi-species preparation including two strains of L. plantarum and P. acidilactici exerted an anti-allergy effect by reducing AHR, decreasing OVA-specific IgE production and Th2 cytokines production (IL-4, IL-5, and IL-13).
Lactobacillus species have been widely evaluated for their efficacy to reduce AHR in an OVA-induced allergy model. However, their efficacy in anti-allergy is both species- and strain-dependent. Forsythe et al. (2007) showed that Lactobacillus reuteri but not L. salivarius reduced AHR.23 In the study, L. reuteri supplementation reduced influx of inflammatory cells in the BALF and decreased levels of MCP-1, TNF-α and IL-13, but L. reuteri supplementation did not affect Th1 responses. Another study showed Lactobacillus acidophilus supplementation effectively reduced eosinophil infiltration in BALF and lowered serum IgE production. IL-4 and IL-5 production was reduced whereas IFN-γ levels were enhanced.24 However, L. acidophilus did not significantly reduce AHR in the OVA-sensitized mice.24 Previous studies also reported that L. plantarum effectively reduced allergic responses in OVA-sensitized mice. For example, heat-killed L. plantarum KTCT 3014 reduced IgE production, Th2 cytokines production (IL-4 and IL-5) and enhanced Th1 cytokine IFN-γ levels, however, the Th1 associated IgG2a secretion was lowered.25 In another study, L. plantarum K37 effectively reduced AHR and lowered production of serum and OVA-specific IgE production.6 Th2 cytokines including IL-4, IL-5 and IL-13 were reduced in the L. plantarum K37 supplemented group. IgG2a production as in the present study was enhanced. From the above studies, it is clear that the anti-allergy effects of Lactobacilli are both species- and strain-dependent.
P. acidilactici has not been reported for anti-allergy effects in an OVA-induced airway allergy model before. A potential role of P. acidilactici for anti-allergy effects is that two strains P. acidilactici H0709 and P. acidilactici NHNM1 were able to suppress histamine release from ionomycin-stimulated basophils.26 A P. acidilactici strain R037 suppressed experimental autoimmune encephalomyelitis by increasing CD4+ Interleukin (IL)-10-producing cells.27 IL-10 has shown to down-regulate excessive Th2 responses in airway inflammation.28,29 To the best of our knowledge, this is the first study to report potential anti-allergy effects of P. acidilactici in an asthmatic allergy model. This P. acidilactici strain was previously selected in a preliminary in vitro and in vivo screen for anti-allergy effects (data not shown). It is possible that P. acidilactici PA320 in the lactobacillus multi-species preparation exert the anti-allergy effects via IL-10 or IL-10 producing regulatory cells. Further investigation for its regulation on the specific population of regulatory cells could be performed for greater understanding.
Conflict of interestThe authors declare no conflicts of interest.
We thank New Bellus Enterprises Co. Ltd. for the production of the test sample LMP.