INTRODUCTION
Many controlled trials have demonstrated that local sublingual-swallow specific immunotherapy (SLIT) is clinically effective in the treatment of selected patients with allergic rhinitis, conjunctivitis and asthma1,2 and an increasing number of observational works reported favourable outcomes in atopic dermatitis (AD)3,4.
Immunotherapy for allergic diseases has been classified as Biological Response Modifier (BRM) and not within any pharmaceutical category because of its multiple effects including the correction of TH2/TH1 imbalance, the down-modulation of T-lymphocyte response, the reduction of the mucosal hyperreactivity etc.1. Furthermore, all the various effects resulting from an effective immunotherapy have been referred, until now, exclusively to mechanisms driven by the specificity of the allergen that is the allergen interaction with cells bearing specific receptors such as IgE for Be cells and the T cell Receptor (TcR) for TH2 cells.
The recognised partial contribution of hemopoietic precursor cells to the allergic inflammation5-7 and the recent report that SLIT is capable of reducing the increased CD34 + peripheral traffic during the treatment of allergic patients8, nevertheless, raised the question as to whether allergen-specific immunotherapy could affect such an immature cells that, given the ontogenetic stage, still do not express the antigen/allergen specific receptors.
One can therefore envisage4 that two different line of mechanisms could act, one allergen-specific following the model of oral tolerance induced with low-dose of antigen (development of allergen-specific suppressor-T clones)9, and the other allergen unspecific, induced by the side-biological activity of the allergens themselves3,4. The House Dust Mite (HDM) major antigen Der p2 is indeed an allergen but works also in a lectin-like manner4,10 as the cytokine interleukin 1 beta (IL1β)11,12 that is capable of inducing the expression of adhesion molecules on human endothelial cells13.
Because of the shared biological activity, the hypothesis has been entertained that the allergen extract, locally administered, could induce an over-expression of cellular adhesion molecules (CAMs) on the vessels of the oral mucosa, thus allowing a subtractive mechanism of the inflammatory circulating cells (physical immune deviation)3,4,8.
This qualitative study has been designed to verify whether mite antigens are capable of modulating CAMs expression on human endothelial cells.
MATERIALS AND METHODS
Slices 2 mm thin of human umbilical cord vein have been used as source of endothelial cells. Specimens were short-term cultured in sterile ready to use tissue medium complete with foetal bovine serum and gentamicin (Amniomed, Euroclone Ltd., Devon, UK) without (control) and with different amounts of House Dust Mite Dermatophagoides pteronyssinus purified major antigens Der p1 and Der p2 for 8 h and then frozen in liquid nitrogen until processed. Der p1 was used at the concentration of 200 μg/ml whereas Der p2 was employed at 30, 200 and 350 μg/ml.
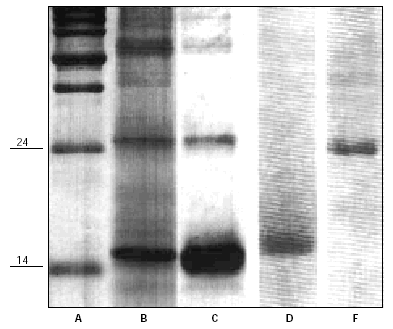
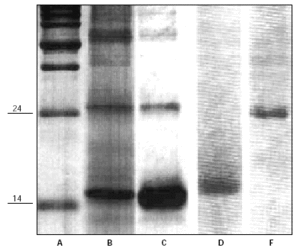
Purified antigens were a generous gift of Dr. Igino Spadolini (Anallergo SrL, Florence, Italy) and of Dr. Jose Carreira (ALK-Abellò, Madrid, Spain) (fig. 1). Antigens were used at high dosages to be sure of identify, if present, the potential stimulating activity.
Figure 1.--SDS-PAGE analysis of Dermatophagoides Pteronyssinus major allergens. Lane A: molecular weight markers (Kilodaltons). Lanes B and C: purified extracts enriched of major antigens Der p1 (Mw 24) and Der p2 (Mw 14) (Anallergo SrL, Florence, Italy). Lane D and F: purified Der p2 (Mw 14) and Der p1 (Mw 14) (ALK-Abellò, Madrid, Spain).
Immunohistochemistry
Umbilical vein serial cryostatic sections (4 μm) were fixed in absolute acetone for 10 min and processed by indirect avidin biotin immunoperoxidase (IIP) method for murine IgG (Vectastain, Vector, Burlingame, CA, USA) for the localisation of the antigens ICAM-1 and VCAM-1. To detect ICAM-1 (CD54) we employed the murine MAB anti-CD54, clone HA58, isotype IgG1 and to detect VCAM-1 (CD106) the murine MAB anti-CD106, clone 51-10C9, isotype IgG1, both from PharMingen, CA, USA. Both primary antibodies were used at the final concentration of 0.5 μg/ml as optimal working dilution. IIP negative controls were represented by sections without primary antibody incubation. Slides were developed with aminoethylcarbazole as the chromogenic substrate, counterstained with Mayer's hematoxylin (Sigma Chem. Co., Milan, Italy) and read by two observers, in blind.
RESULTS
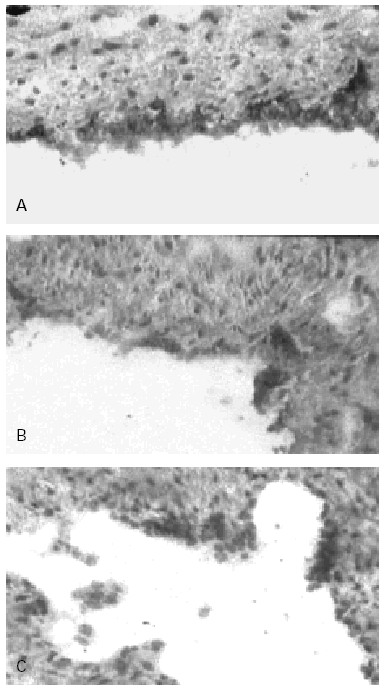
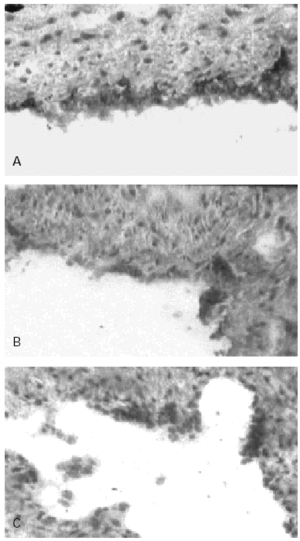
In control sections, no staining of the endothelium with anti-CD106 MAB (VCAM-1) was observed while a low reactivity with the anti-CD54 MAB (ICAM-1) was detectable, confirming that the latter molecule is constitutionally expressed at low level on human endothelial cells (HEC). In control sections furthermore most of the cells forming the endothelial lining appeared flat in shape, with slightly represented cytoplasm. The endothelial staining with the anti-CD54 MAB to ICAM-1 was significantly increased in sections of the cultures incubated with Der p1 (fig. 2A); cultures stimulated with Der p2 at concentration 30 μgr/ml showed only a low-to-moderate increased expression of ICAM-1 (fig. 2B) when compared to the negative control (fig. 2C). The higher concentrations of Der p2 used did not resulted into further increase of ICAM-1 expression as for this antigen the activation mechanism does not appear to be regulated in a dose-dependent fashion.
Figure 2.--Human Umbilical Cord Vein (HUCV) sections short-term cultured with mite antigens Der p1 or Der p2. Indirect Immunoperoxidase (IIP) for the endothelial localisation of ICAM-1 molecules. (A): ICAM-1 up-regulation induced by Der p1; all the endothelial cells of the luminal border appear extensively stained (200x). (B): low-to-moderate positivity for ICAM-1 induced by Der p2 (100 x). (C): control section cultured without allergens (100x).
Morphologically, the endothelial cells stimulated with Der p1 and positive for ICAM-1 and VCAM-1 (figs. 2A and 3A), appeared augmented in height and with strongly immunoreactive cytoplasm, a shape resembling High Endothelial Vein Cells.
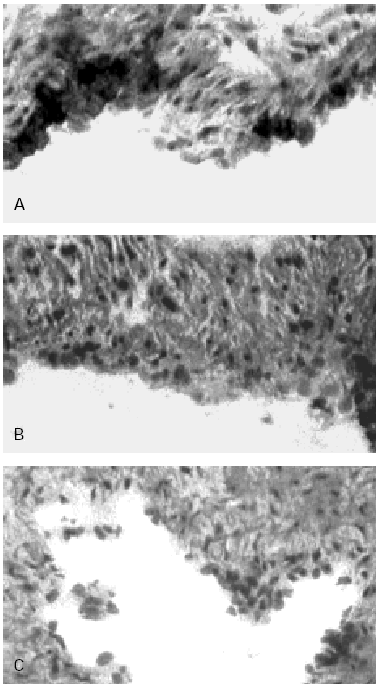
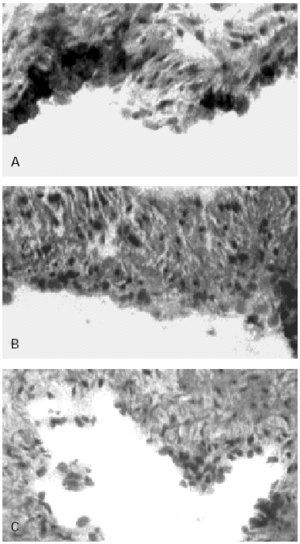
VCAM-1 while undetectable in the control sections of human endothelium (fig. 3C) was clearly expressed in sections of umbilical cord incubated with Der p1 (fig. 3A). In the latter in fact some clusters of intensely stained cells could be observed (fig. 3A).
Figure 3.--HUCV sections cultured with Mite antigens Der p1 and Der p2. IIP for the endothelial localisation of VCAM-1 molecule. (A): VCAM-1 expression induced by Der p1; cluster of well stained endothelial cells are detectable (400x). (B): poorly valuable reactivity for VCAM-1 induced by Der p2 (150x). (C): Negative control section (150x).
The immunoreactive pattern of VCAM-1 was different from ICAM-1 since the former consisted in scattered cluster of positive cells (fig. 3A) while in the latter the reactivity involved continuously all the endothelial cells (fig. 2A).
Der p2 stimulation appeared poorly if not effective on the VCAM-1 expression (fig. 3B).
DISCUSSION
The oral sublingual route of specific immunotherapy administration is a reliable model to investigate how SIT can affect allergic inflammation.
The low amount of protein used, the lack of absorption and the prolonged persistence of the fed antigen at the level of the oral mucosa15 strongly suggested that at least some of the SLIT mechanisms of action have to occur locally.
Among the others, the restoration of TH2/TH1 balance (phenomenon known as immune deviation) is a main proved effect of specific immunotherapy but there is controversy as to whether these changes occurs and if they are detectable in peripheral blood16. In fact the mechanism by which the minimal amount of allergen can influence, at-distance from the site of administration, changes at the peripheral sites of allergic inflammation still remains a matter of debate. A local subtractive mechanism of the circulating TH2 and other inflammatory cells thus preventing the homing of such cells to periphery, could be one of the mechanisms contributing to the immune-deviation.
Another important clinical outcome of SIT, strongly implying the natural history of allergic diseases, is the proved capability of preventing the development of new sensitisation in early-treated patients. The possibility that IgE synthesis can take place not only in germinal centres of lymph node but also at the level of peripheral inflamed mucosae has been recently recognised17 Plasmacytoid monocytes (PM), acting as antigen-presenting-cells, have been now identified also at mucosal sites of direct antigen exposure beside in bone marrow and central lymphoid tissue18. Finally, the compelling evidence that human precursor cells (HPC) are important contributors to allergic inflammation5-8,19,20, raise the question as to whether the generation/expansion of Be clones responsible of the new allergen-sensitisation which develop at the periphery could be impaired by the "subtractive mechanism".
Adhesion molecules are critical in the regulation of leukocytes traffic and in the initiation and progression of tissue allergic inflammation, nevertheless only recently the different phases of such an inflammation have been investigated in relation to the sequential expression of vascular CAMs and to the different type of cells recruited.
Experimental studies on the early and late phases of inflammation, have demonstrated that the cellular adhesion to endothelium is mediated by at least two or more overlapping and redundant CAMs types, i.e. homing receptors specific to ICAM-1 and VCAM-1. In particular, evidence have been provided that this phenomenon appear to be biphasic. While at the very early phase of inflammation the recruitment of less specialised (Neutrophils)21-23 or less mature cells (Lymphoblasts)24 is driven mainly by ICAM-1, at later phases activated eosinophils and mature T-cells (T-Lymphocytes)23,24 are captured by VCAM-1. The study of the vascular CAMs ontogeny in human skin, furthermore, indicates that ICAM-1 and P-selectin are the first and the most represented adhesion molecules on foetal skin vasculature whereas VCAM-1 and E-selectin expression occur at low level at significantly olderage25.
All together the above mentioned evidence suggest that the very early phase of the inflammation may be poorly selective, with a recruitment of less mature and less specialised cells mediated mainly by ICAM-1 which is constitutionally expressed on the endothelium and thus susceptible of rapid up-regulation. The late phase, distinctive of the allergic inflammation, appears dominated by committed and mature cells that use preferentially the homing receptor specific to VCAM-1.
Such emerging model of a sequential cellular recruitment at inflammation sites and the preliminary finding of the present work could suggest to appraise another effect of the sublingual immunotherapy recently described. SLIT has been reported to be capable of reducing the increased CD34 + precursor cells traffic in the peripheral blood of allergic patients8. Since CD34 + HPC, among the redundant repertoire of homing receptors, use preferentially the CD11a and CD49d molecules (counterligands for ICAM-1 and VCAM-1) for trans-endothelial migration26,27, the observed reduction of the HPC peripheral traffic in SLIT treated subjects may be linked to a capturing of these immature cells in the vessels of the oral mucosa where the administered allergenic extract has induced an over-expression of addressins.
In conclusion, data of the present qualitative study showing an endothelial up-regulation of ICAM-1 and the de-novo expression of VCAM-1 induced by the house-dust-mite antigens investigated are consistent with the proposed model of a local recruitment of circulating inflammatory cells at the sites of allergen extracts administration.
Results are furthermore supported by the recent demonstration that Dermatophagoides farinae activates nuclear factor (NF)-Kappa B that is one of the transduction pathways for vascular adhesion molecules transcription28-30.