Chronic spontaneous urticaria (CSU) is a frequent clinical entity that often presents a diagnostic and therapeutic challenge.
ObjectiveTo explore the degree of agreement that exists among the experts caring for patients with CSU diagnosis, evaluation, and management.
MethodsAn online survey was conducted to explore the opinions of experts in CSU, address controversial issues, and provide recommendations regarding its definition, natural history, diagnosis, and treatment. A modified Delphi method was used for the consensus.
ResultsThe questionnaire was answered by 68 experts (dermatologists, allergologists, and primary care physicians). A consensus was reached on 54 of the 65 items posed (96.4%). The experts concluded that CSU is a difficult-to-control disease of unpredictable evolution. Diagnostic tests should be limited and based on clinical history and should not be indiscriminate. Autoinflammatory syndromes and urticarial vasculitis must be ruled out in the differential diagnosis. A cutaneous biopsy is only recommended when wheals last more than 24h, to rule out urticarial vasculitis. The use of specific scales to assess the severity of the disease and the quality of life is recommended. In patients with severe and resistant CSU, second-generation H1-antihistamines could be used at doses up to four times the standard dose before giving second-line treatments. Omalizumab is a safe and effective treatment for CSU that is refractory to H1-antihistamines treatment. In general, diagnosis and treatment recommendations given for adults could be extrapolated to children.
ConclusionsThis work offers consensus recommendations that may be useful in the management of CSU.
Urticaria is a cutaneous disease characterised by the appearance of wheals, angio-oedema, or both.1 It is estimated that 8–20% of the population is likely to experience at least one episode of acute urticaria,1,2 and 0.6–1.8% of the population is likely to experience an episode of chronic urticaria during their life. Chronic urticaria, defined as daily or almost daily outbreaks for more than six weeks, may significantly affect the quality of patients’ life and their daily activities and performance.1 Pruritus, interference with sleep, lesions on visible body parts, or difficulty moving the joints of the ankles or the hands can become disabling.
In recent years, advances in the understanding of the pathogenesis of urticaria, updates of management guidelines, improved diagnostic techniques, and the advent of highly relevant therapeutic advances have highlighted the morbidity of chronic urticaria. According to the latest classification of the European Academy of Allergy and Clinical Immunology (EAACI), chronic urticaria comprises inducible urticarias (among which are the physical urticarias) and chronic spontaneous urticaria (CSU) (Table 1). The treatment of CSU can be frustrating for both patients and physicians because the lesions may persist, despite the use of existing treatments.2 Recently, the use of omalizumab, a humanised monoclonal anti-IgE antibody, has been approved for the treatment of CSU. It is capable of controlling the symptoms of CSU in a significant number of patients who are non-responders to high dose antihistamines in a monotherapy regimen or in combination with other drugs.1 Its safety and efficacy have been demonstrated in randomised placebo-controlled clinical trials where it was used when antihistamines (H1 and H2) or leukotriene receptor antagonists (LTRA) were proven to be ineffective.4–6
Classification of chronic urticaria.
| Subtypes of chronic urticaria | |
|---|---|
| Chronic spontaneous urticaria | Inducible urticaria |
| Spontaneous appearance of wheals, angio-oedema, or both lasting ≥6 weeks | Physical urticaria − Symptomatic dermographism − Cold urticaria − Delayed pressure urticaria − Solar urticaria − Heat urticaria − Vibratory angio-oedema Cholinergic urticaria Contact urticaria Aquagenic urticaria |
This document addresses the management of CSU by focusing on its natural history and diagnosis, its clinical evaluation and follow-up, and its treatment in routine clinical practice. After consensus was reached, some recommendations that can facilitate the clinical management of this disease were developed. Our aim was to study the degree of consensus that the recently published European guidelines have among the specialists in Dermatology and Allergy. The analysis of the results of this work will contribute to the current knowledge of a frequent disease in emergency rooms, primary care, internal medicine, and allergology and dermatology. This analysis will contribute to the proper management of the patient from the start of clinical occurrence, achieving thus better control of the disease and reducing its social and economic impact.
Materials and methodsThe Delphi methodA modified Delphi method was used in this study.7 According to this method, the opinions of a panel of experts were anonymously requested through the use of an online questionnaire in two rounds of voting. The questionnaire consisted of assertions or items that addressed some controversial aspects about CSU and were scored according to the degree of the panelists’ agreement or disagreement with them. The results obtained were statistically analysed, and the results of the first vote were circulated among the participants. The items on which there was no consensus were re-circulated and subjected to a second round of voting. In this manner, the experts could reconsider their responses in the light of the pooled results. The results obtained in this second round were statistically analysed to determine which issues had finally achieved a sufficient degree of consensus among the experts, and whether or not they were in agreement or in disagreement with each item presented.
Phases of the processInitially, a scientific committee was established, by inviting the participation of a small group of prestigious experts in the management of CSU. This scientific committee was responsible for designing the questionnaire, which contained the relevant aspects for discussion and to reach a consensus. Subsequently, a panel of 68 experts who would be responsible for the voting was established.
The online questionnaire was distributed to all of the panellists. The items were evaluated by means of a Likert-type ordinal scale of nine points (minimum 1: complete disagreement, and maximum 9: complete agreement), according to the format developed by the UCLA-RAND Corporation.7 The 9 possible responses were divided into three groups (1–3=disagreement; 4–6=neither agreement nor disagreement; and 7–9=agreement). The questionnaire offered the participants the possibility to include free observations or to propose new items. The items on which there was no consensus in the first round were subjected to a second evaluation by all of the panellists in the second round of the process. Before the second round of evaluation, the panellists were informed of the results of the first round and of the comments contributed. The questionnaire results were analysed by external reviewers. The scientific committee discussed the results and composed the final document. To simplify the results, a summary table of the most important consensual items was prepared with 15 recommendations and conclusions. This summary was reviewed and approved by all of the members of the scientific committee.
Statistical analysis and interpretation of resultsThe resulted score of each item is shown in tables as the median and interquartile range (IQR). The percentage of panellists in favour of the majority response is shown, i.e., the percentage of panellists whose score was in any of the three-point groups containing the median.1–9 An item was considered consensual in agreement if the median of the scores fell within the 7–9 group and was considered consensual in disagreement if the median fell within the 1–3 group. For an item to be considered consensual, the number of panellists who voted outside of the 1–3 or 7–9 regions had to be less than 1/3 of the total and the IQR had to be ≤4. The same criteria were applied to discriminate between the consensual items in the second round.
ResultsThe questionnaire consisted of 56 items divided into four blocks (Tables 2–5). In the first round of evaluation, consensus was reached on 44 of the 56 questions (78.5%). Twelve questions on which there was no consensus were subjected to a second round of evaluation. After the second round, a consensus was reached on ten of them. Subsequently, after two rounds of evaluation, it was possible to reach a consensus on 54 of the 56 proposed items (96.4%). All of the consensual items were agreed upon completely by all the participants without any issues of disagreement being of concern. The most important consensual items are summarised in the 15 recommendations and conclusions below and also in Table 6.
- 1.
CSU is a cutaneous disease characterised by the daily or almost daily appearance of transitory pruritic wheals for at least six weeks. It is a difficult-to-control disease, its evolution is unpredictable, with remissions and spontaneous recurrences, and it is not known whether or not current treatments can modify its natural history.
- 2.
CSU can generally be diagnosed by means of the clinical history and physical examination. The diagnostic tests to be performed on patients with suspected CSU should be limited and based on the clinical history. The process of diagnosing CSU in children is similar to the adult one.
- 3.
CSU fairly often is associated with some type of chronic inducible urticaria, especially dermographism, cholinergic urticaria, and delayed-pressure urticaria. Non-steroidal anti-inflammatory drugs can frequently trigger or exacerbate CSU.
- 4.
In our setting, there is no need for a systematic search for infections (such as Helicobacter pylori, Giardia lamblia, and Anisakis simplex) in the process of diagnosing CSU.
- 5.
Autoinflammatory syndromes and urticarial vasculitis must be ruled out in the differential diagnosis of CSU.
- 6.
In general, it is not necessary to perform a cutaneous biopsy for the diagnosis of CSU. It is advisable to perform a cutaneous biopsy only when the wheals last more than 24h to rule out or confirm urticarial vasculitis.
- 7.
Determining the severity of CSU is primarily based on the intensity of the symptoms and signs: intensity of itching and number of wheals. Currently, no validated biomarkers exist for monitoring the severity of CSU.
- 8.
To evaluate the severity of CSU and the response to treatment, it is advisable to use scales such as the UAS7 (Urticaria Activity Score used for one week) and the AAS (Angioedema Activity Score).
- 9.
During the follow-up of patients with CSU, it is recommended to perform an evaluation of the quality of life through appropriate questionnaires, such as the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) and the Angioedema Quality of Life Questionnaire (AE-Q2oL).
- 10.
Second-generation H1-antihistamines should be used in the treatment of CSU. In patients with severe and resistant CSU, these could be used at doses up to four times the standard dose before giving second-line treatments. One antihistamine can be switched to another to individually determine which is the most effective and safest for each patient. Continuous use is more beneficial and effective than use on demand.
- 11.
The use of short cycles of corticosteroids is useful in the treatment of the exacerbations of CSU. Prolonged use of corticosteroids is not recommended. The use of cyclosporine may be considered, although it is not indicated for CSU and the renal function and blood pressure, among other functions adversely affected by corticosteroids, should be monitored. There is insufficient evidence to recommend the use of anti-leukotrienes in the treatment of CSU.
- 12.
Omalizumab is the only treatment approved by the EMA and FDA as an additional treatment for CSU in adults and adolescents (12 years and older) who are refractory to H1-antihistamines. It is a safe and effective treatment.
- 13.
The recommended dose of omalizumab for the treatment of refractory CSU is 300mg subcutaneously every four weeks, independently of the body mass index or the levels of IgE. Following discontinuation of treatment with omalizumab, most patients with CSU progressively return to their initial symptomatology. Rebound effect has not been described.
- 14.
Few studies have evaluated the efficacy of the treatment of chronic urticaria in the paediatric population, so in general the recommendations given to adults are extrapolated to children. Any indication of treatment with systemic corticosteroids, omalizumab, or cyclosporine in patients younger than 12 years of age should be done with caution and after an individualised analysis.
- 15.
During pregnancy and lactation, urticaria must be controlled using the minimum dose of medication that can be effective. Hydroxyzine is contraindicated in pregnancy. During lactation, in the event that the use of H1-antihistamines is necessary, loratadine or cetirizine are recommended.
Results of block 1. Natural history and diagnosis.
| Median (IQR) | Degree of agreement | Result | |
|---|---|---|---|
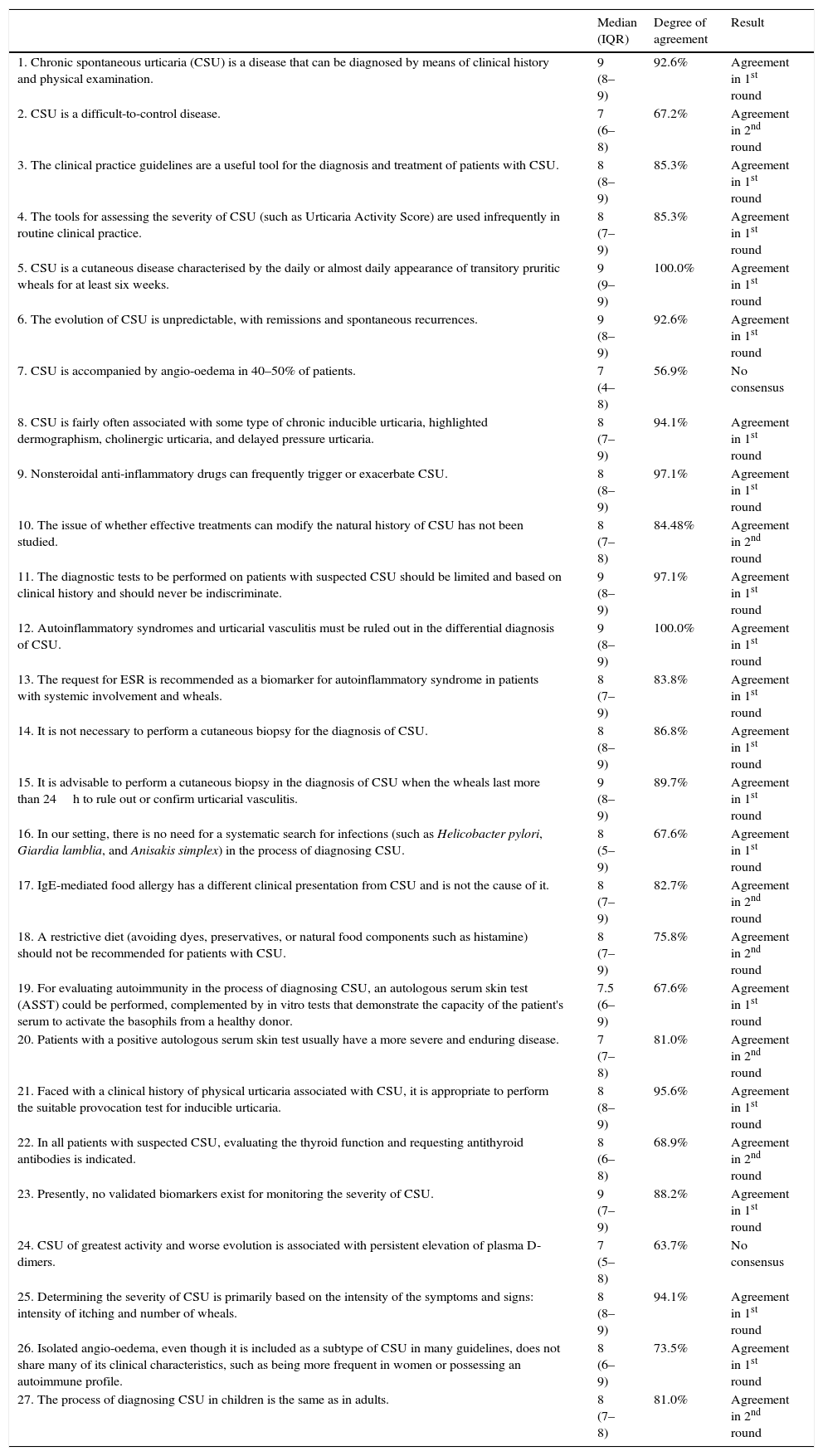
| 1. Chronic spontaneous urticaria (CSU) is a disease that can be diagnosed by means of clinical history and physical examination. | 9 (8–9) | 92.6% | Agreement in 1st round |
| 2. CSU is a difficult-to-control disease. | 7 (6–8) | 67.2% | Agreement in 2nd round |
| 3. The clinical practice guidelines are a useful tool for the diagnosis and treatment of patients with CSU. | 8 (8–9) | 85.3% | Agreement in 1st round |
| 4. The tools for assessing the severity of CSU (such as Urticaria Activity Score) are used infrequently in routine clinical practice. | 8 (7–9) | 85.3% | Agreement in 1st round |
| 5. CSU is a cutaneous disease characterised by the daily or almost daily appearance of transitory pruritic wheals for at least six weeks. | 9 (9–9) | 100.0% | Agreement in 1st round |
| 6. The evolution of CSU is unpredictable, with remissions and spontaneous recurrences. | 9 (8–9) | 92.6% | Agreement in 1st round |
| 7. CSU is accompanied by angio-oedema in 40–50% of patients. | 7 (4–8) | 56.9% | No consensus |
| 8. CSU is fairly often associated with some type of chronic inducible urticaria, highlighted dermographism, cholinergic urticaria, and delayed pressure urticaria. | 8 (7–9) | 94.1% | Agreement in 1st round |
| 9. Nonsteroidal anti-inflammatory drugs can frequently trigger or exacerbate CSU. | 8 (8–9) | 97.1% | Agreement in 1st round |
| 10. The issue of whether effective treatments can modify the natural history of CSU has not been studied. | 8 (7–8) | 84.48% | Agreement in 2nd round |
| 11. The diagnostic tests to be performed on patients with suspected CSU should be limited and based on clinical history and should never be indiscriminate. | 9 (8–9) | 97.1% | Agreement in 1st round |
| 12. Autoinflammatory syndromes and urticarial vasculitis must be ruled out in the differential diagnosis of CSU. | 9 (8–9) | 100.0% | Agreement in 1st round |
| 13. The request for ESR is recommended as a biomarker for autoinflammatory syndrome in patients with systemic involvement and wheals. | 8 (7–9) | 83.8% | Agreement in 1st round |
| 14. It is not necessary to perform a cutaneous biopsy for the diagnosis of CSU. | 8 (8–9) | 86.8% | Agreement in 1st round |
| 15. It is advisable to perform a cutaneous biopsy in the diagnosis of CSU when the wheals last more than 24h to rule out or confirm urticarial vasculitis. | 9 (8–9) | 89.7% | Agreement in 1st round |
| 16. In our setting, there is no need for a systematic search for infections (such as Helicobacter pylori, Giardia lamblia, and Anisakis simplex) in the process of diagnosing CSU. | 8 (5–9) | 67.6% | Agreement in 1st round |
| 17. IgE-mediated food allergy has a different clinical presentation from CSU and is not the cause of it. | 8 (7–9) | 82.7% | Agreement in 2nd round |
| 18. A restrictive diet (avoiding dyes, preservatives, or natural food components such as histamine) should not be recommended for patients with CSU. | 8 (7–9) | 75.8% | Agreement in 2nd round |
| 19. For evaluating autoimmunity in the process of diagnosing CSU, an autologous serum skin test (ASST) could be performed, complemented by in vitro tests that demonstrate the capacity of the patient's serum to activate the basophils from a healthy donor. | 7.5 (6–9) | 67.6% | Agreement in 1st round |
| 20. Patients with a positive autologous serum skin test usually have a more severe and enduring disease. | 7 (7–8) | 81.0% | Agreement in 2nd round |
| 21. Faced with a clinical history of physical urticaria associated with CSU, it is appropriate to perform the suitable provocation test for inducible urticaria. | 8 (8–9) | 95.6% | Agreement in 1st round |
| 22. In all patients with suspected CSU, evaluating the thyroid function and requesting antithyroid antibodies is indicated. | 8 (6–8) | 68.9% | Agreement in 2nd round |
| 23. Presently, no validated biomarkers exist for monitoring the severity of CSU. | 9 (7–9) | 88.2% | Agreement in 1st round |
| 24. CSU of greatest activity and worse evolution is associated with persistent elevation of plasma D-dimers. | 7 (5–8) | 63.7% | No consensus |
| 25. Determining the severity of CSU is primarily based on the intensity of the symptoms and signs: intensity of itching and number of wheals. | 8 (8–9) | 94.1% | Agreement in 1st round |
| 26. Isolated angio-oedema, even though it is included as a subtype of CSU in many guidelines, does not share many of its clinical characteristics, such as being more frequent in women or possessing an autoimmune profile. | 8 (6–9) | 73.5% | Agreement in 1st round |
| 27. The process of diagnosing CSU in children is the same as in adults. | 8 (7–8) | 81.0% | Agreement in 2nd round |
Results of block 2. Clinical evaluation and monitoring in routine clinical practice.
| Median (IQR) | Degree of agreement | Result | |
|---|---|---|---|
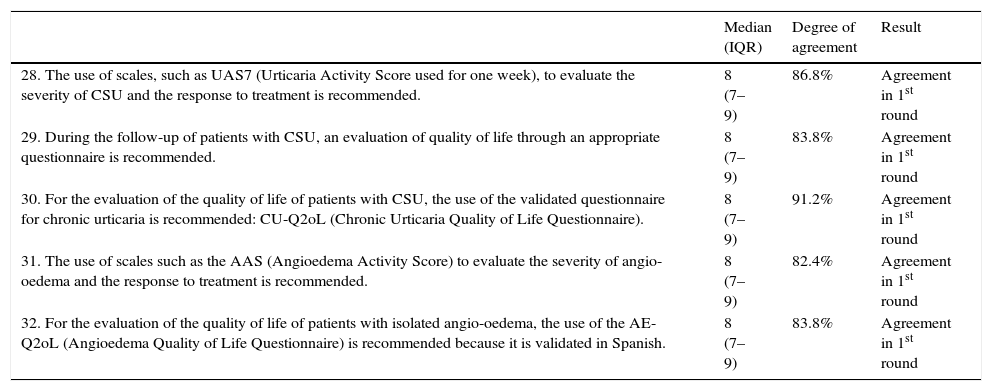
| 28. The use of scales, such as UAS7 (Urticaria Activity Score used for one week), to evaluate the severity of CSU and the response to treatment is recommended. | 8 (7–9) | 86.8% | Agreement in 1st round |
| 29. During the follow-up of patients with CSU, an evaluation of quality of life through an appropriate questionnaire is recommended. | 8 (7–9) | 83.8% | Agreement in 1st round |
| 30. For the evaluation of the quality of life of patients with CSU, the use of the validated questionnaire for chronic urticaria is recommended: CU-Q2oL (Chronic Urticaria Quality of Life Questionnaire). | 8 (7–9) | 91.2% | Agreement in 1st round |
| 31. The use of scales such as the AAS (Angioedema Activity Score) to evaluate the severity of angio-oedema and the response to treatment is recommended. | 8 (7–9) | 82.4% | Agreement in 1st round |
| 32. For the evaluation of the quality of life of patients with isolated angio-oedema, the use of the AE-Q2oL (Angioedema Quality of Life Questionnaire) is recommended because it is validated in Spanish. | 8 (7–9) | 83.8% | Agreement in 1st round |
Results of block 3: treatment.
| Median (IQR) | Degree of agreement | Result | |
|---|---|---|---|
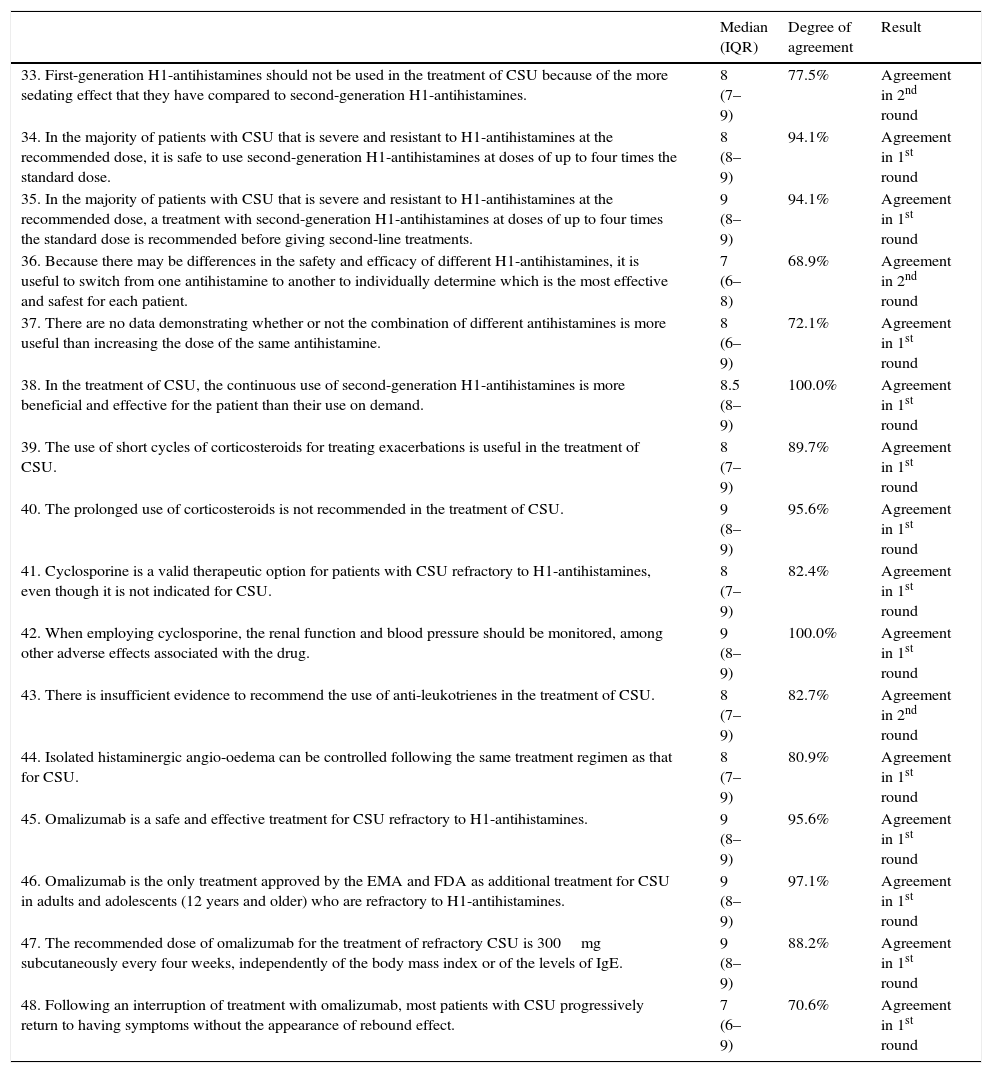
| 33. First-generation H1-antihistamines should not be used in the treatment of CSU because of the more sedating effect that they have compared to second-generation H1-antihistamines. | 8 (7–9) | 77.5% | Agreement in 2nd round |
| 34. In the majority of patients with CSU that is severe and resistant to H1-antihistamines at the recommended dose, it is safe to use second-generation H1-antihistamines at doses of up to four times the standard dose. | 8 (8–9) | 94.1% | Agreement in 1st round |
| 35. In the majority of patients with CSU that is severe and resistant to H1-antihistamines at the recommended dose, a treatment with second-generation H1-antihistamines at doses of up to four times the standard dose is recommended before giving second-line treatments. | 9 (8–9) | 94.1% | Agreement in 1st round |
| 36. Because there may be differences in the safety and efficacy of different H1-antihistamines, it is useful to switch from one antihistamine to another to individually determine which is the most effective and safest for each patient. | 7 (6–8) | 68.9% | Agreement in 2nd round |
| 37. There are no data demonstrating whether or not the combination of different antihistamines is more useful than increasing the dose of the same antihistamine. | 8 (6–9) | 72.1% | Agreement in 1st round |
| 38. In the treatment of CSU, the continuous use of second-generation H1-antihistamines is more beneficial and effective for the patient than their use on demand. | 8.5 (8–9) | 100.0% | Agreement in 1st round |
| 39. The use of short cycles of corticosteroids for treating exacerbations is useful in the treatment of CSU. | 8 (7–9) | 89.7% | Agreement in 1st round |
| 40. The prolonged use of corticosteroids is not recommended in the treatment of CSU. | 9 (8–9) | 95.6% | Agreement in 1st round |
| 41. Cyclosporine is a valid therapeutic option for patients with CSU refractory to H1-antihistamines, even though it is not indicated for CSU. | 8 (7–9) | 82.4% | Agreement in 1st round |
| 42. When employing cyclosporine, the renal function and blood pressure should be monitored, among other adverse effects associated with the drug. | 9 (8–9) | 100.0% | Agreement in 1st round |
| 43. There is insufficient evidence to recommend the use of anti-leukotrienes in the treatment of CSU. | 8 (7–9) | 82.7% | Agreement in 2nd round |
| 44. Isolated histaminergic angio-oedema can be controlled following the same treatment regimen as that for CSU. | 8 (7–9) | 80.9% | Agreement in 1st round |
| 45. Omalizumab is a safe and effective treatment for CSU refractory to H1-antihistamines. | 9 (8–9) | 95.6% | Agreement in 1st round |
| 46. Omalizumab is the only treatment approved by the EMA and FDA as additional treatment for CSU in adults and adolescents (12 years and older) who are refractory to H1-antihistamines. | 9 (8–9) | 97.1% | Agreement in 1st round |
| 47. The recommended dose of omalizumab for the treatment of refractory CSU is 300mg subcutaneously every four weeks, independently of the body mass index or of the levels of IgE. | 9 (8–9) | 88.2% | Agreement in 1st round |
| 48. Following an interruption of treatment with omalizumab, most patients with CSU progressively return to having symptoms without the appearance of rebound effect. | 7 (6–9) | 70.6% | Agreement in 1st round |
Results of block 4: special situations.
| Median (IQR) | Degree of agreement | Result | |
|---|---|---|---|
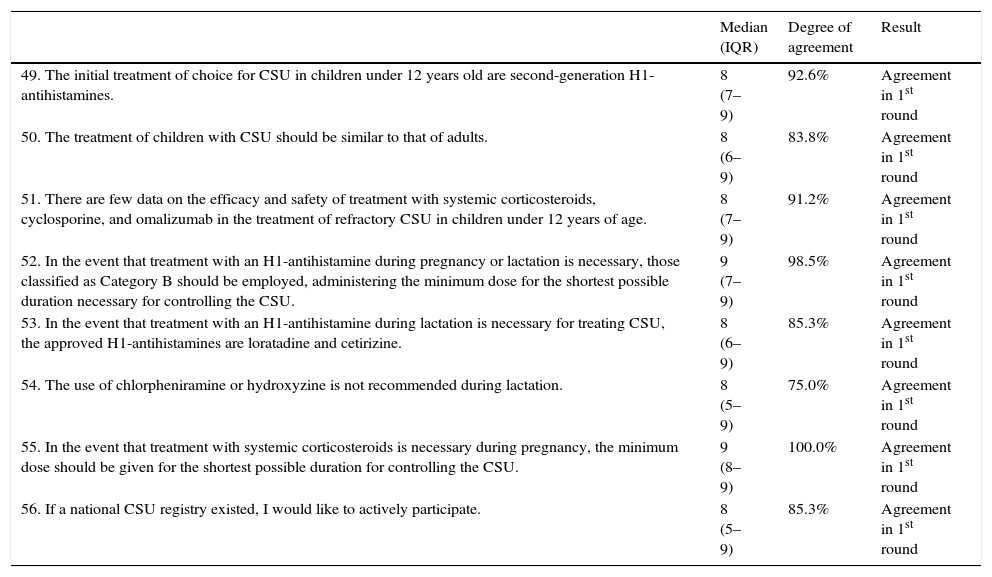
| 49. The initial treatment of choice for CSU in children under 12 years old are second-generation H1-antihistamines. | 8 (7–9) | 92.6% | Agreement in 1st round |
| 50. The treatment of children with CSU should be similar to that of adults. | 8 (6–9) | 83.8% | Agreement in 1st round |
| 51. There are few data on the efficacy and safety of treatment with systemic corticosteroids, cyclosporine, and omalizumab in the treatment of refractory CSU in children under 12 years of age. | 8 (7–9) | 91.2% | Agreement in 1st round |
| 52. In the event that treatment with an H1-antihistamine during pregnancy or lactation is necessary, those classified as Category B should be employed, administering the minimum dose for the shortest possible duration necessary for controlling the CSU. | 9 (7–9) | 98.5% | Agreement in 1st round |
| 53. In the event that treatment with an H1-antihistamine during lactation is necessary for treating CSU, the approved H1-antihistamines are loratadine and cetirizine. | 8 (6–9) | 85.3% | Agreement in 1st round |
| 54. The use of chlorpheniramine or hydroxyzine is not recommended during lactation. | 8 (5–9) | 75.0% | Agreement in 1st round |
| 55. In the event that treatment with systemic corticosteroids is necessary during pregnancy, the minimum dose should be given for the shortest possible duration for controlling the CSU. | 9 (8–9) | 100.0% | Agreement in 1st round |
| 56. If a national CSU registry existed, I would like to actively participate. | 8 (5–9) | 85.3% | Agreement in 1st round |
Conclusions and recommendations.
| 1. CSU is a cutaneous disease characterised by the daily or almost daily appearance of transitory pruritic wheals for at least six weeks. It is a difficult-to-control disease, its evolution is unpredictable, with remissions and spontaneous recurrences, and it is not known whether or not current treatments can modify its natural history. |
| 2. CSU can generally be diagnosed by means of the clinical history and physical examination. The diagnostic tests to be performed on patients with suspected CSU should be limited and based on the clinical history and should never be indiscriminate. The process of diagnosing CSU in children is the same as the one in adults. |
| 3. CSU is fairly often associated with some type of chronic inducible urticaria, especially dermographism, cholinergic urticaria, and delayed-pressure urticaria. Non-steroidal anti-inflammatory drugs are factors that can frequently trigger or exacerbate CSU. |
| 4. In our setting, there is no need for a systematic search for infections (such as Helicobacter pylori, Giardia lamblia, and Anisakis simplex) in the process of diagnosing CSU. |
| 5. Autoinflammatory syndromes and urticarial vasculitis must be ruled out in the differential diagnosis of CSU. |
| 6. In general, it is not necessary to perform a cutaneous biopsy for the diagnosis of CSU. It is advisable to perform a cutaneous biopsy only when the wheals last more than 24h to rule out or confirm urticarial vasculitis. |
| 7. Determining the severity of CSU is primarily based on the intensity of the symptoms and signs: intensity of itching and number of wheals. Currently, no validated biomarkers exist for monitoring the severity of CSU. |
| 8. To evaluate the severity of CSU and the response to treatment, it is advisable to use scales such as the UAS7 (Urticaria Activity Score used for one week) and the AAS (Angioedema Activity Score). |
| 9. During the follow-up of patients with CSU, is recommended to perform an evaluation of the quality of life through appropriate questionnaires, such as the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) and the Angioedema Quality of Life Questionnaire (AE-Q2oL). |
| 10. Second-generation H1-antihistamines should be used in the treatment of CSU. In patients with severe and resistant CSU, these could be used at doses up to four times the standard dose before giving second-line treatments. One antihistamine can be switched to another to individually determine which is the most effective and safest for each patient. Continuous use is more beneficial and effective than use on demand. |
| 11. The use of short cycles of corticosteroids is useful in the treatment of the exacerbations of CSU. Prolonged use of corticosteroids is not recommended. The use of cyclosporine may be considered, although it is not indicated for CSU and the renal function and blood pressure, among other functions adversely affected by corticosteroids, should be monitored. There is insufficient evidence to recommend the use of anti-leukotrienes in the treatment of CSU. |
| 12. Omalizumab is the only treatment approved by the EMA and FDA as an additional treatment for CSU in adults and adolescents (12 years and older) who are refractory to H1-antihistamines. It is a safe and effective treatment of CSU refractory to H1-antihistamines. |
| 13. The recommended dose of omalizumab for the treatment of refractory CSU is 300mg subcutaneously every four weeks, independently of the body mass index or the levels of IgE. Following an interruption of treatment with omalizumab, most patients with CSU progressively return to having symptoms without rebound effect. |
| 14. Few studies have evaluated the efficacy of the treatment of chronic urticaria in the paediatric population, so in general the recommendations given to adults are extrapolated to children. Any indication of treatment with systemic corticosteroids, omalizumab, or cyclosporine in patients younger than 12 years of age should be done with caution and after an individualised evaluation. |
| 15. During pregnancy and lactation, urticaria must be controlled using the minimum dose of medication that can be effective. Hydroxyzine is contraindicated in pregnancy. During lactation, in the event that the use of H1-antihistamines is necessary, loratadine or cetirizine are recommended. |
Urticaria is a frequent reason for consultation, not only in dermatology and allergology services, but also in emergency rooms, primary care, and internal medicine. Chronic urticaria, in particular, frequently presents a challenge for these specialists, especially when symptoms are resistant to the first line of treatment. Various aspects of the definition and natural history of CSU, as well as its diagnosis and treatment, were addressed by this study. Consensus was achieved on most of the questions addressed. This eminently practical approach may help resolve doubts and serve as a guide to the specialists involved in the management of this disease.
The first block of the consensual items addressed the natural history and diagnosis of CSU. The panel's definition of CSU is in line with the definition given in the most recent guidelines. In these guidelines, the appearance of angio-oedema is included in the definition of urticaria, comprising of patients who display wheals with or without angio-oedema and patients with angio-oedema only. Approximately 40% of the patients with CSU have associated angio-oedema.1 However, the clinical experience of the panellists may be different, because an agreement on the item that was related to this point was not reached. According to the experts’ opinion, isolated angio-oedema although it is included as a subtype of CSU in many guidelines, does not share many of its clinical characteristics, such as being more frequent in women or possessing an autoimmune profile.
For most patients with CSU there is no evidence of any triggering factor.3 However, the panellists believed that non-steroidal anti-inflammatory medications could trigger or exacerbate CSU, as has also been described in the literature.8 In some observational studies, the consumption of dyes, preservatives, or natural components of foods (such as histamine itself) have been reported as aggravating factors.9 However, a recent placebo-controlled study with 11 such additives has shown no effect.10 Considering this ultimate study, the panellists reached consensus that a restrictive diet (avoiding dyes, preservatives, etc.) should not be recommended in patients with CSU. There was also agreement that CSU is fairly often associated with a type of chronically induced urticaria, (dermographism, cholinergic urticaria, and delayed-pressure urticaria were highlighted), which is useful to know to adequately manage this type of patients.
With respect to diagnosis, in the most recent management guidelines for chronic urticaria, it is recommended to make rational use of complementary tests.1,11 The panellists mostly agreed on this aspect, indicating that CSU is a disease that can be diagnosed through the clinical history and physical examination and that diagnostic tests performed on patients with suspected CSU should be limited and based on the clinical history and should never be indiscriminate. The diagnostic process should be similar in both adults and children, according to the panel. Furthermore, with respect to the differential diagnosis of CSU, it was agreed that the autoinflammatory syndromes and urticarial vasculitis should be ruled out and that in the case of a clinical history of physical urticaria being associated with CSU, the appropriate provocation test for inducible urticaria should be performed, according to the recent recommendations.11 It should also be noted that the panel considered that, in general, it is not necessary to perform a cutaneous biopsy for the diagnosis of CSU, although it is advisable to perform one when the wheals last more than 24h, to rule out or confirm urticarial vasculitis. On the other hand, in our setting, there is no need to perform a systematic search for infections or insect infestation (such as H. pylori, G. lamblia, and A. simplex) in the process of diagnosing CSU.
Other practical recommendations that can be extracted from the opinions expressed by the panellists are that in all patients with suspected CSU, evaluation of thyroid function, and tests for detection of antithyroid antibodies are indicated, and moreover that the erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP) are recommended as biomarkers of inflammation in patients with wheals and systemic involvement in the context of autoinflammatory syndrome.
The autologous serum skin test (ASST) is a procedure that, although not generally used, can help demonstrate the autoimmune nature of the CSU.4 The panellists suggested that in order to evaluate autoimmunity in the process of diagnosing CSU, an ASST could be performed, complemented by in vitro tests, which demonstrate the capacity of the patient's serum to activate the basophils from a healthy donor. Patients with a positive ASST typically have a more severe and enduring disease,3 which reflects the opinion of the panel of experts as well.
With respect to the evaluation of severity, some studies have attempted to relate some serum biomarkers with the severity of the disease. For example, in one study, high levels of D-dimer were found in some patients with CSU, and an association was suggested between this finding and a more severe disease, as well as worse response to antihistamines and cyclosporine.12,13 Other biomarkers that are mentioned in some studies are interleukin-6, C-reactive protein, metalloproteinase-9, and complement C3 and C4.3 However, the experts believed that, currently, no validated biomarkers exist for monitoring the severity of CSU, and the determination of the severity of CSU is primarily based on the intensity of symptoms and signs (intensity of the pruritus and number of wheals). Although there are tools, such as the Urticaria Activity Score (UAS),1,11 to evaluate the severity of CSU, the panellists thought that these tools are used infrequently in routine clinical practice.
Block 2 specifically addressed the clinical evaluation and monitoring of patients with CSU in routine clinical practice. Consensus was reached on all items of this block in the first round. It was thought that the CSU evaluation tools in the previous block were underused, therefore their use was recommended in the current block, namely usage of the UAS or UAS7 for one week. The UAS7 (as defined in the European consensus) is a simple questionnaire in which the wheals and pruritus are scored from 0 to 3 (0: none; 3: intense) for seven consecutive days, giving a maximum score of 42. In the event that there is angio-oedema, the use of scales such as the Angioedema Activity Score (AAS) to assess severity and the response to treatment are recommended. This tool evaluates the activity and severity of angio-oedema for seven days through six simple questions. Similarly, the evaluation of the quality of life through an appropriate questionnaire, specifically the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL), which has been adapted and validated in Spanish, is recommended.14 For the evaluation of the quality of life of patients with isolated angio-oedema, the use of the Angioedema Quality of Life Questionnaire (AE-Q2oL) was suggested as well. The most recent management guidelines also recommend using the UAS7 activity questionnaire and the CU-Q2oL and AE-Q2oL quality of life questionnaires.1
Block 3 addressed the treatment of CSU and, as in the previous blocks, consensus was achieved on all of the items evaluated. Antihistamines (1st and 2nd generation H1) are the first treatment of choice in the treatment of CSU.1 They are more effective than placebo in the treatment of CSU, although there is insufficient evidence to say whether or not any of the available treatments is superior to the others.15 Various practical recommendations for their use are issued in this consensus. The panel reached a consensus that first-generation H1-antihistamines should not be used in the treatment of CSU because of the more sedating effect they have, when compared to the second-generation. This recommendation was reached in the second round, even though it is a point that is also recommended in the guidelines with a high level of evidence.1 With respect to the dosage of antihistamines, in patients with severe urticaria where the standard dose of the antihistamine is not effective, there is evidence that increasing the standard dose up to four times can be useful.16 Thus, the panellists recognised that in most patients with severe CSU that is resistant to the standard dose, second-generation H1-antihistamines can be used at doses of up to four times the standard dose before giving second-line treatments. Caution should be exercised when these medications are used in combination with certain categories of medications and in the presence of co-existing diseases. The decision to treat with a higher dose than the recommended one remains a matter of expert opinion only. Moreover, the response and tolerance to different antihistamines may be different even in the same patient.16 Therefore, the experts agreed that it might be useful to switch from one antihistamine to another to individually determine which is the most effective and safest for each patient. On the contrary, they agreed that no data show whether or not the combination of different antihistamines is more useful than increasing the dose of the same antihistamine. They also agreed that the continuous use of second-generation H1-antihistamines is more beneficial and effective than their use PRN (pro re nata).17
In the final questions of block 3, a consensus was reached on clinical aspects related to possible treatments other than with antihistamines. Corticosteroids are recommended in short cycles, and it was recommended that the use of cyclosporine could be considered,18 although it is worth noting that cyclosporine use is not indicated for CSU and that renal function and blood pressure, among other functions that can be adversely affected by this drug, must be monitored. The efficacy of LTRA in CSU has recently been revised, discouraging its use as monotherapy.19 According to this revision, its use as adjunctive therapy to antihistamines may be justified in some situations, but more studies are needed to clarify its role in CSU because the evidence in this regard is scarce and of poor quality.18,19
The recommendation made by the panellists for the use of innovative treatments such as omalizumab should be noted. There is very solid evidence of its safety and efficacy in the treatment of allergic asthma,20 and it has recently been approved for use in the treatment of CSU.21 Omalizumab is the only treatment approved by the European Medicines Agency (EMA)21 and the Food and Drug Administration (FDA)22 as additional treatment for CSU in adults and adolescents (12 years and older) who are refractory to H1-antihistamines, a fact with which the panellists are in agreement. Omalizumab is indicated for patients with CSU who already receive antihistamines but whose symptoms are not well controlled by these medications. In this study, omalizumab was considered a safe and effective treatment for CSU refractory to H1-antihistamines, which is in line with the clinical trials that led to its approval. In two phase III, randomised, placebo-controlled trials,4,5 the safety and efficacy of omalizumab were demonstrated in patients with CSU who remained symptomatic despite H1-antihistamine treatment with the approved dose. A third controlled study6 primarily evaluated the safety of omalizumab in patients with CSU who remained symptomatic despite treatment with H1-antihistamines with a dose up to four times the approved one and H2-antihistamines and/or treatment with LTRA. In total, the three trials included 975 patients aged between 12 and 75 years (mean age 42.3 years), with 259 men and 716 women. In all of these trials, omalizumab showed a good safety profile. There was no evidence that safety was altered when combined with antihistamines or LTRA, which is in accordance with its known safety profile in allergic asthma. Unlike its use for allergic asthma, where the dose of omalizumab is adjusted to the IgE (immunoglobulin E) level and body weight, the recommended dose of omalizumab for the treatment of refractory CSU is 300mg subcutaneously every four weeks, independently of the body mass index or of the level of IgE.23 As for the duration of the treatment, the experience from clinical trials for CSU indicated treatment longer than six months is limited.23 In a phase III study,6 after the discontinuation of treatment, most patients slowly relapsed within a period of 10 weeks. This clinical experience is consistent with the responses of the panellists, who agreed that after an interruption of omalizumab treatment, most of the patients with CSU progressively return to having symptoms, however no rebound effect has been observed. There is evidence that the resumption of treatment with omalizumab is effective once again. In a retrospective study of 25 patients with CSU or chronic inducible urticaria who had responded completely to omalizumab and had relapsed after its suspension, the efficacy and safety of retreatment were assessed. There was a complete response after treatment without relevant adverse effects in all of the patients.24 In another retrospective study, omalizumab was reintroduced in 20 patients, achieving control in 18 of them (90%).25 Although evidence for the efficacy of retreatment with omalizumab in CSU seems to be scarce, such use is contemplated in its data sheet.21
Finally, in the items of the last block, the treatment of CSU in special situations was discussed, specifically in children and in women during pregnancy and lactation. Few studies have evaluated the efficacy of the treatment of chronic urticaria in the paediatric population; therefore, in general, the recommendations given to adults are extrapolated to children.26,27 Currently, cetirizine could be used in children beginning at six months of age; cetirizine, loratadine, desloratadine, and ebastine could be used after two years; and rupatadine after six years of age.3 A randomised double-blind study has recently shown that rupatadine is effective and safe in the treatment of CSU in children between 2 and 12 years of age.28 Compared to other treatments, our panel believed that there are few data on the efficacy and safety of systemic corticosteroids, cyclosporine, and omalizumab in the treatment of refractory CSU in children under 12 years old. Therefore, the use of this treatment in patients of this age group should be done with caution and every case should be considered individually.3 Treatment of CSU in pregnancy does not differ radically from treatment of non-pregnant patients, however during pregnancy and lactation, urticaria should be treated by using the least possible of the required dose of medication to achieve effective control.29 There are no antihistamines in category A of the FDA; thus, the panellists concluded that women should use second-generation category B antihistamines during pregnancy. Antihistamines of this category B are loratadine, cetirizine, levocetirizine, and chlorpheniramine; however, the latter is not recommended for use during lactation because of its sedative effects on the newborns.3 Hydroxyzine is contraindicated in pregnancy (category C of the FDA) because it crosses the placenta barrier, reaching a foetal concentration above that of the mother and because in animal studies it has been shown to cause toxicity.30 If treatment with an H1-antihistamine during lactation is necessary, then the H1-antihistamines loratadine and cetirizine are recommended.
It is interesting to note that if there were a national registry of CSU, then most of the panellists would like to actively participate. A registry of this type, for monitoring the use of biological treatments such as omalizumab, could be interesting. The experience of biological treatment registries already exists in dermatology, such as the Spanish Registry of Systemic Treatments in Psoriasis (Registro Español de tratamientos sistémicos en psoriasis – BIOBADADERM), which has provided relevant clinical information.31
In summary, in this document, consensus was reached on some basic aspects related to the definition and natural history of CSU, and a few guidelines with regard to the necessary actions to be adopted are issued, when evaluating and treating patients with this disease. We trust that these aspects, agreed upon by experts, can serve as guidelines to the clinician to meet the challenge that often exists in the management of CSU.
Conflict of interestsAll of the signatories belong to the consulting group named the Research Group in Chronic Urticaria (Grupo de Investigación en Urticaria Crónica – GRUC), coordinated by Drs. Ana Giménez-Arnau and Marta Ferrer and sponsored by Novartis Pharmaceuticals.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
This work was sponsored by Novartis Pharmaceuticals. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
The authors would like to make special mention and show their appreciation to the panellists (allergologists, dermatologists, and primary-care physicians) who participated in the voting according to the Delphi method to produce this document: Miquel Armengot-Carbó, María Luisa Baeza Ochoa De Ocariz, Isabel Bielsa Marsol, Teresa Caballero, Paloma Campo, Alfonso Carreño Rojo, Pedro Carretero Anibarro, José Manuel Comas Samper, Ignacio Dávila González, Diego De Argila Fernández-Duran, Pablo De La Cueva Dobao, Carlos De La Torre Fraga, Luis Javier Del Pozo Hernando, Francisco José Esteban González, Emilia Fernández López, Marta Ferrer Royo, Pere Gaig Jané, Manuel Galán Gutiérrez, M. Mar Garcés Sotillos, Juan García Gavín, Gloria Garnacho, Yolanda Gilaberte Calzada, Elena Godoy Gijón, Pilar Gómez Avivar, Eloina González Mancebo, Ruperto González Pérez, Julio Antonio Heras Hitos, Elena Hernández Gª De La Barrera, Marcos Hervella Garcés, Pilar Iriarte Sotés, Mario Linares Barrios, Ramón Lleonart Bellfill, Ángel López Ávila, Jose Luis López Estebaranz, Lluis Marqués Amat, Juan Márquez Enriquez, Jose Manuel Mascaró Galy, Almudena Mateu Puchades, Anna Medvedeva, Francisco Javier Miquel Miquel, Estrella Montero Navarro, Manuel Moragon Gordon, Jose María Olaguibel Rivera, David Palacios Martínez, Javier Pedraz Muñoz, Javier Pellegrini Belinchón, Lorenzo Pérez García, Amalia Pérez Gil, Jesús Luis Prieto Andrés, Joaquin Quiralte Enriquez, Ángeles Revert, José Manuel Ródenas López, Mercedes Rodríguez, Mercedes Rodríguez Serna, Ricardo Ruiz-Villaverde, Esther Serra Baldrich, José Suárez Hernández, Ana Isabel Tabar Purroy, Miguel Ángel Tejedor Alonso, Jose Ignacio Torné Gutiérrez, Sergio Vañó Galvan, Hugo Vázquez Veiga, Beatriz Veleiro Pérez, David Vidal Sarró, Jaime Vilar Alejo, María del Carmen Vizán De Uña, Violeta Zaragoza Ninet, and José Manuel Zubeldia Ortuño.
We thank Nature Publishing Group Iberoamérica and Dr. Pablo Rivas for the editorial support provided.