Eosinophilic esophagitis (EoE) is characterized by esophageal dysfunction and, histologically, by eosinophilic inflammation. There is not a clear etiologic treatment. Biopsies analysis using plant histology methods may show callose and pollen tubes in the esophageal mucosa. Component-resolved diagnosis (CRD) with microarrays could detect possible allergens involved and indicate an elimination diet and allergen immunotherapy (AIT).
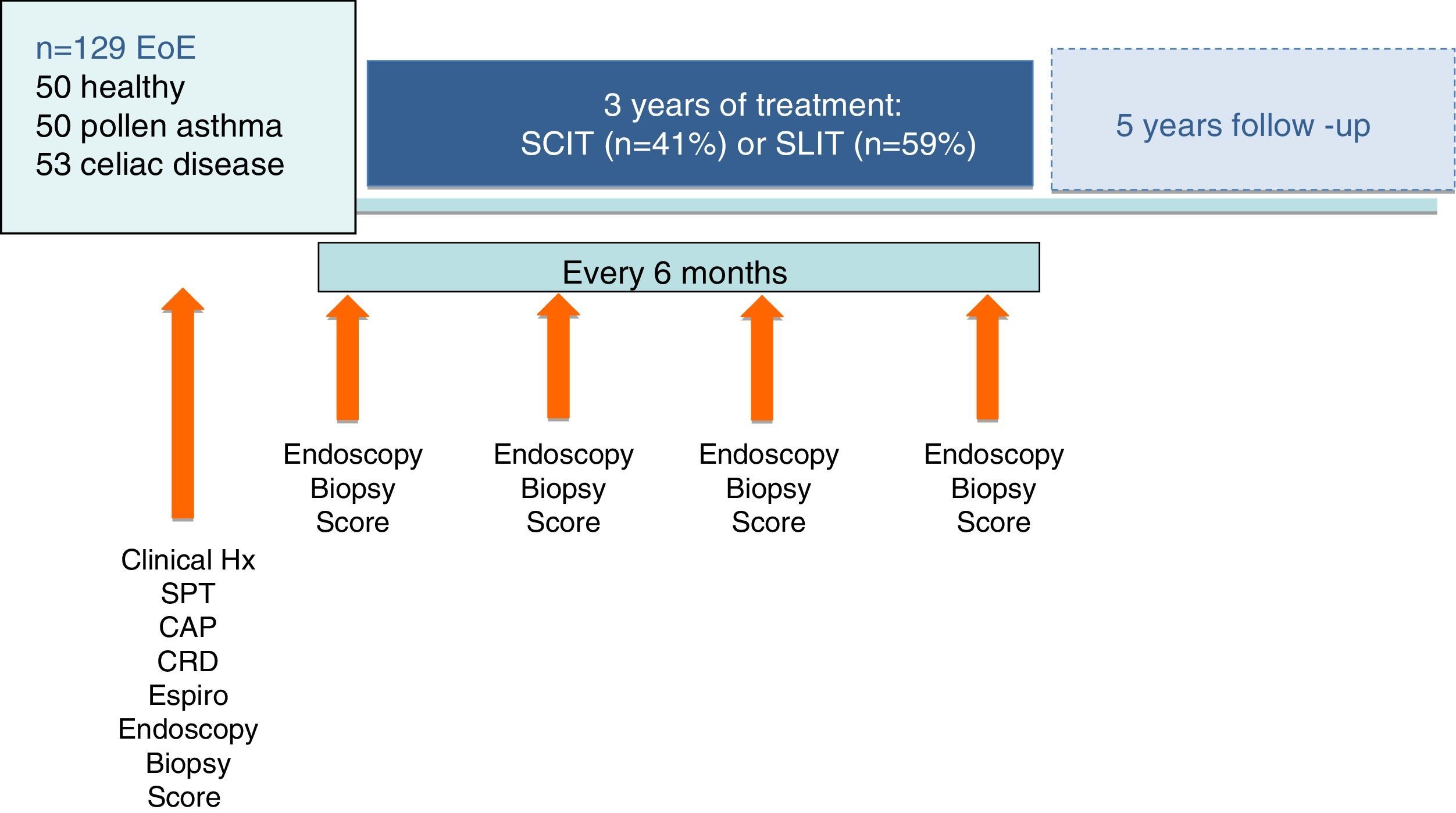
MethodsOne hundred and twenty-nine patients with EoE were tested for environmental and food allergens. CRD, histological and botanical analysis were performed. Clinical scores and endoscopic biopsy were performed every six months for three years. Fifty healthy patients, 50 asthmatics due to pollen, and 53 celiac disease patients were included as comparison groups. CRD-directed AIT was administered in 91 EoE patients and elimination diet in 140 patients (87 EoE and all 53 CD patients).
ResultsCRD detected allergen hypersensitivity in 87.6% of patients with EoE. The predominant allergens were grass group 1 (55%), lipid transfer proteins (LTP) of peach and mugwort, hazelnuts and walnuts. Callose from pollen tubes was found in 65.6% of biopsies. After CRD-guided elimination diet and/or AIT, 101 (78.3%) EoE patients showed significant clinical improvement (p<0.017) and 97 (75.2%) were discharged (negative biopsy, no symptoms, no medication) without relapse.
AIT-treated patients had better outcomes (odds ratio 177.3, 95% CI 16.2–1939.0).
ConclusionCRD-directed AIT and/or elimination diet was efficient in treating EoE patients and was well tolerated.
Eosinophilic esophagitis (EoE) is a chronic atopic disease of unclear etiology with characteristic histology – dense eosinophilic leukocyte infiltration. The estimated prevalence is 0.4% in children and adults,1 and men are preferentially affected.1–3 Previously considered a food allergy, genetic, molecular, cellular, animal and translational studies have shown that, in EoE, exposure to allergens leads to a complex, coordinated type 2 inflammatory reaction causing eosinophil influx.1–6 Mucosal barrier dysfunction due to desmoglein dysregulation,7 eotaxin-3 disturbance, thymic stromal lymphopoietin (as in rhinitis and allergic asthma),8 IL13 and filaggrin, and CAPN14, has been reported.1,2 Persistent EoE may lead to esophageal fibrosis, in which TGF-beta and TNF-alpha are involved.7,8
Many EoE patients present rhinoconjunctivitis, atopic dermatitis and associated asthma, in addition to dysphagia and food impaction, and 87% are sensitive to aeroallergens.5 The phenotype is the same whether or not patients are sensitized to foods, and EoE exacerbations are often seasonal.3,5,6,9 Children with EoE are more often sensitized to foods6 and adults to airborne allergens.1,2,6,9
The utility of allergy testing in the etiologic diagnosis of EoE is unclear: some reports state they lack utility10 and others that they are necessary and that component-resolved diagnosis (CRD)-directed elimination diets are effective.11–13
In a pilot study of 67 EoE patients, we found CRD-guided diagnosis and allergen immunotherapy (AIT) showed a high percentage of patients were sensitized to environmental allergens, especially pollens, and that after three years of CRD-guided diet restriction and AIT, EoE significantly improved.13
We hypothesized that, as the esophageal and bronchial mucosa share the same embryonic origin,14 they might respond with similar inflammatory mechanisms to environmental and food allergenic stimuli and that asthma due to allergens and esophagitis may have an equivalent response to AIT.
Some reports suggest that so-called “immunotherapy” with food, (in fact, the induction of tolerance, not to be confused with AIT), is not indicated in EoE. Meta-analyses have been based on very few valid studies. Lucendo15 selected only three of the 118 reports considered due to their methodology, excluding two good studies in which AIT with aeroallergens improved patients, and concluded that AIT was related to EoE in 2.7% of patients, although the endoscopic study before AIT was not clear. In an EoE patient hypersensitive to a food, the induction of tolerance with the same food could present problems, as may any desensitization technique, albeit controlled.
Most studies have shown that IgE is not important in EoE16 and that food triggering EoE can only be identified by an elimination diet with subsequent reintroduction controlled by endoscopy and histology, which has limited treatment with specific immunotherapy. However, no study has ruled out a role of allergen-directed specific IgE antibodies, in addition to other inflammatory mechanisms in EoE.
We also hypothesized that the inflammatory response of the esophageal mucosa in patients with high levels of antibodies to pollen allergens and worsening seasonal EoE may be due to swallowing airborne pollen and the intrusion into the esophageal mucosa of pollen tubes emitted after pollen germination that encounter a pH and humidity resembling the stigma at pollination,17,18 which might be facilitated by desmoglein deficit.7
We aimed to fulfill the classical Koch-Henle postulates,19 which show that a causal agent must be present in each case, must not be found randomly in other diseases or healthy controls, and can be identified in all damaged tissues.
The objectives of this study were: to obtain an accurate etiological diagnosis of EoE using standard allergy tests and CRD; to demonstrate a pathogenic role for environmental allergens in EoE using human and plant histology; and, to evaluate the effectiveness of CRD-guided specific AIT and/or elimination diet.
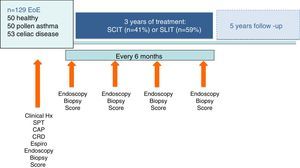
Material and methodsDesignWe made an observational, longitudinal study to compare the effectiveness and safety profile of CRD-guided specific AIT and/or elimination diet with usual EoE maintenance therapy over a five-year period of real time analysis (o real world study). All suitable patients with EoE from two hospitals and 21 primary care centers in the autonomic community of Castile and Leon, Spain, were identified from practice databases and invited to participate in the study. Inclusion criteria were a diagnosis of EoE (symptoms of food impaction and >15 eosinophils/field on endoscopic biopsy) followed by our Gastroenterology Service from 2010, with a proton pump inhibitor (PPI) trial to confirm the diagnosis20 and treated for at least nine months with conventional therapy without clinical improvement.
We finally included 129 patients diagnosed with EoE. These patients were provided with sufficient information to decide whether to receive usual therapy (oral corticosteroids, proton-pump inhibitors, six-food diet) or CRD-guided diet and AIT. The patient information sheet and informed consent form, and a further informed consent for histological biopsy study, were approved by the University Hospital Rio Hortega Ethics Committee.
The intent-to-treat (ITT) population was defined as all patients who received at least one prescription of study medication (e.g., AIT, diet or usual EoE maintenance therapy). The primary effectiveness analysis (PEA) population was defined as all ITT patients with a dysphagia score of >9 at baseline.
Three comparative groups were included: fifty randomly-selected healthy blood donors with digestive or allergic disease ruled out; fifty randomly-selected asthmatic patients from our data base of patients with pollen allergy (positive skin prick, specific IgE to pollens and spirometric criteria of moderate-asthma); fifty-three pediatric patients with clinical, serological and biopsy-confirmed celiac disease (CD) from our Pediatric Department and that of the Rio Carrion Hospital, Palencia, included because reports show co-comorbidity between EoE and CD.13 CD patients were included as, like other authors, we have observed the coexistence of susceptibility with EoE in these patients.21
TreatmentPersonalized therapy was administered as required in each case according to the clinical history, in vivo, endoscopic, and serological tests and CRD.
Directed elimination: elimination of foods identified as allergens: prick test >10mm×10mm, IgE class 4 to the food or >15 ISAC standardized units (ISU). The diet was explained by a nutritionist and was directed only against the food detected by CRD, except for wheat allergens, in which case barley and rye were also eliminated from the diet.
AIT alone or AIT plus elimination diet: when causal foods and also environmental allergens were detected. Specific AIT (subcutaneous and sublingual, ALK-Abelló SA) was administered in EoE patients sensitized to allergens with licensed AIT and CD patients with positive IgE to grass pollens and/or wheat allergens. Patients chose subcutaneous or sublingual AIT according to personal criteria and availability to attend our hospital for subcutaneous AIT. Children received sublingual AIT.
Compliance with and safety of AIT was assessed at six-monthly visits in which prick tests, specific IgE measurement (InmunoCAP Thermofisher, Upssala, Sweden) and endoscopy were repeated in patients and controls who agreed. Patients included and the personalized therapies administered are shown in Fig. 1.
After two years follow up and after suspending AIT but continuing with diet recommendations, a favorable evolution was defined as no symptoms requiring medication and a decrease in eosinophil infiltrate to <5 eosinophils in biopsies. Clinical discharge was defined as no symptoms or need for medication and a negative biopsy. Relapses were evaluated every six months for two years after clinical discharge. Esophageal dilation was not necessary in any case.
Clinical evaluationsClinical evaluations and esophageal biopsies were made every six months in May/June and November/December. Gastrointestinal (dysphagia, heartburn, stomach upset, vomiting, constipation, diarrhea, failure to thrive) and allergic (rhinitis, asthma, dermatitis, anaphylaxis) histories were taken and an examination made. A visual analogic dysphagia score (0–10) was administered in EoE and CD patients.
Patients were also telephoned each month to determine whether they had experienced any serious adverse events or non-serious adverse AIT reactions. A face-to-face visit was made every six months. The study team monitored all hospital admissions, outpatient and emergency department visits, as well as primary care data (including all healthcare contacts, out-of-hours activity and prescriptions of oral steroids or other drugs) via the electronic health records.
In vivo tests: skin prick test (SPT) to 42 aeroallergens and food allergens including grass pollen, trees, shrubs, mites and other arthropods, epithelia from eight animals, and 15 foods, including Anisakis. Endoscopic diagnosis including esophageal biopsy (positive=≥15 eosinophils/field) was made in all EoE and CD patients, 15 healthy controls and 14 pollen allergy patients who agreed to biopsy.
In vitro tests: allergen specific E immunoglobulin (IgE) measured by ImmunoCAP and CRD to 112 recombinant and native allergens (Thermofisher®, Sweden).
Human and plant histology was made of esophageal biopsies from EoE, CD, pollen allergy and control patients (211 biopsies) to determine callose, a carbohydrate polymer β1–3 linked glucose that appears during tissue development, (pollen tube), mechanical stress and the response to pathogenic and symbiotic infections.17 All biopsies were blinded and independently evaluated by ≥4 pathologists. Stains used in human biopsies do not normally detect plant structures.17 Therefore, plant histology was made using Sirofluor (aniline blue fluorochrome).18
The best histological resolution under light microscopy was epoxy-resin embedded pieces, sectioned in 1–2μm semithin sections, and stained with toluidine blue.
Pollen, spores and other plant elements in the esophageal mucosa were evaluated using scanning electron microscopy (SEM) in patients and healthy controls.
Statistical analysisThe t-test was used to compare means and the Chi-square test for tests of independence. The odds ratio of the treatment effects on outcomes was calculated.
The odds ratio expressed in the results was calculated as the ratio between the odds of achieving better EoE control with AIT and/or diet compared with continuing with usual care. The ratios were adjusted for any imbalances between the treatment arms in certain key characteristics.
ResultsDescriptive analysis129 patients with EoE and all controls completed the entire protocol. The mean age was 35±16.24 years in EoE patients, 26±10.26 in asthma patients, 6±4.41 in CD patients and 32±11.07 in controls. EoE patients (46 female) and CD patients (41 male, 12 female) were predominantly male.
The only significant sociodemographic difference was that CD patients were younger (p<0.05), as CD is usually diagnosed in childhood. Fourteen EoE patients had concomitant CD and seven CD patients concomitant EoE. Sixty-two EoE patients had allergic symptoms (11 asthma, 42 rhinitis and eight anaphylaxis).
CRD detected significantly more allergen positivity than IgE (p<0.001). Significant associations were found between EoE and pollen allergy (p=0.000), and other digestive diseases (p=0.004).
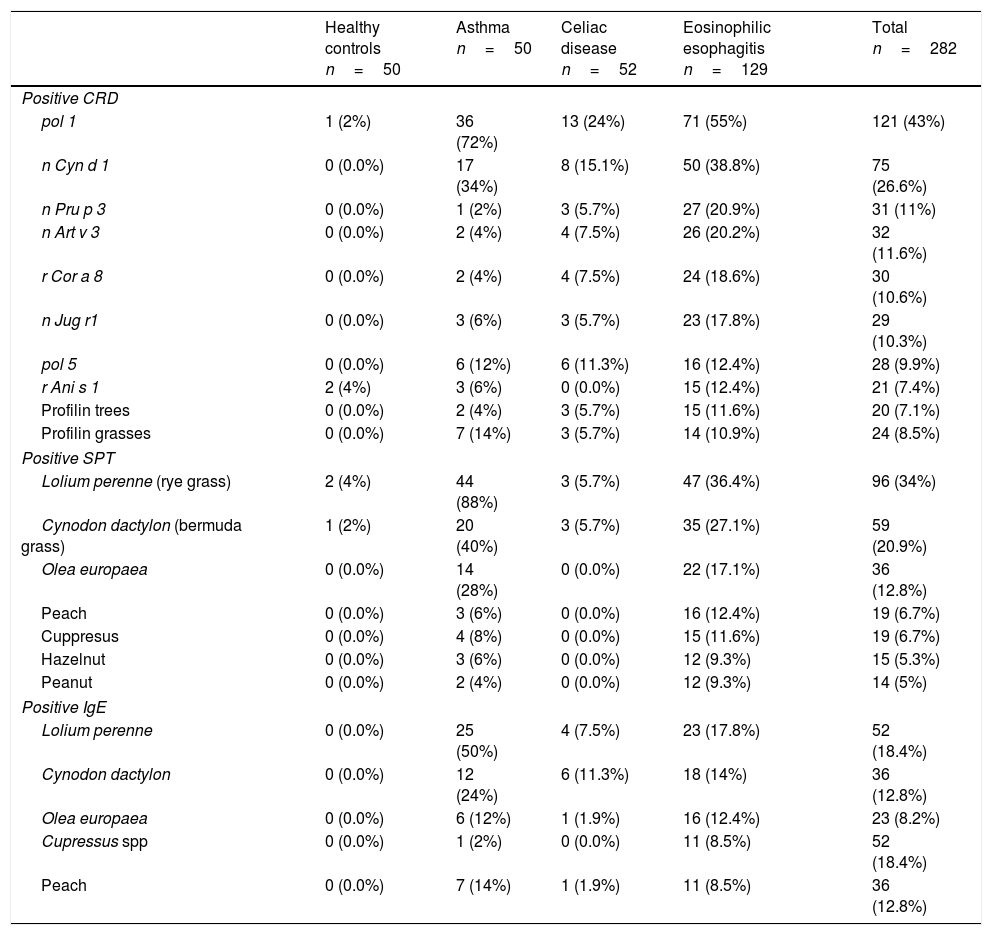
CRD detected sensitization to recombinant group 1 grass allergens (71 [55%] EoE patients, 13[24%] CD), Cynodon dactylon nCyn d1 (50 [38.8%] EoE, 8 [15.1%] CD), lipid transfer proteins (LTPs) of peach (27 [20.9%] EoE, 3 [5.7%] CD), mugwort (26 [20.2%] EoE, 4 [7.5%] CD), hazelnut (24 [18.6%] in EoE, 4 [7.5%] CD), nut albumin (23 [17.8%] EoE, 3 [5.7%] CD), profilin (15 [11.6%] EoE, 3 [5.7%] CD), and serine protease of Anisakis (15 [12.4%] EoE). Six CD patients responded to wheat LTP.
Prick tests showed 47 (36.4%) EoE patients were sensitized to Lolium perenne (rye grass pollen) and 35 (27.1%) to Cynodon dactylon (Bermuda grass pollen).
Specific IgE showed 23 (17.8%) EoE patients were positive to Lolium pollens, 18 (14%) to Cynodon and 11 (8.5%) to peach (Table 1).
Specific allergens detected by CRD, SPT and IgE in > 10% of patients tested.
| Healthy controls n=50 | Asthma n=50 | Celiac disease n=52 | Eosinophilic esophagitis n=129 | Total n=282 | |
|---|---|---|---|---|---|
| Positive CRD | |||||
| pol 1 | 1 (2%) | 36 (72%) | 13 (24%) | 71 (55%) | 121 (43%) |
| n Cyn d 1 | 0 (0.0%) | 17 (34%) | 8 (15.1%) | 50 (38.8%) | 75 (26.6%) |
| n Pru p 3 | 0 (0.0%) | 1 (2%) | 3 (5.7%) | 27 (20.9%) | 31 (11%) |
| n Art v 3 | 0 (0.0%) | 2 (4%) | 4 (7.5%) | 26 (20.2%) | 32 (11.6%) |
| r Cor a 8 | 0 (0.0%) | 2 (4%) | 4 (7.5%) | 24 (18.6%) | 30 (10.6%) |
| n Jug r1 | 0 (0.0%) | 3 (6%) | 3 (5.7%) | 23 (17.8%) | 29 (10.3%) |
| pol 5 | 0 (0.0%) | 6 (12%) | 6 (11.3%) | 16 (12.4%) | 28 (9.9%) |
| r Ani s 1 | 2 (4%) | 3 (6%) | 0 (0.0%) | 15 (12.4%) | 21 (7.4%) |
| Profilin trees | 0 (0.0%) | 2 (4%) | 3 (5.7%) | 15 (11.6%) | 20 (7.1%) |
| Profilin grasses | 0 (0.0%) | 7 (14%) | 3 (5.7%) | 14 (10.9%) | 24 (8.5%) |
| Positive SPT | |||||
| Lolium perenne (rye grass) | 2 (4%) | 44 (88%) | 3 (5.7%) | 47 (36.4%) | 96 (34%) |
| Cynodon dactylon (bermuda grass) | 1 (2%) | 20 (40%) | 3 (5.7%) | 35 (27.1%) | 59 (20.9%) |
| Olea europaea | 0 (0.0%) | 14 (28%) | 0 (0.0%) | 22 (17.1%) | 36 (12.8%) |
| Peach | 0 (0.0%) | 3 (6%) | 0 (0.0%) | 16 (12.4%) | 19 (6.7%) |
| Cuppresus | 0 (0.0%) | 4 (8%) | 0 (0.0%) | 15 (11.6%) | 19 (6.7%) |
| Hazelnut | 0 (0.0%) | 3 (6%) | 0 (0.0%) | 12 (9.3%) | 15 (5.3%) |
| Peanut | 0 (0.0%) | 2 (4%) | 0 (0.0%) | 12 (9.3%) | 14 (5%) |
| Positive IgE | |||||
| Lolium perenne | 0 (0.0%) | 25 (50%) | 4 (7.5%) | 23 (17.8%) | 52 (18.4%) |
| Cynodon dactylon | 0 (0.0%) | 12 (24%) | 6 (11.3%) | 18 (14%) | 36 (12.8%) |
| Olea europaea | 0 (0.0%) | 6 (12%) | 1 (1.9%) | 16 (12.4%) | 23 (8.2%) |
| Cupressus spp | 0 (0.0%) | 1 (2%) | 0 (0.0%) | 11 (8.5%) | 52 (18.4%) |
| Peach | 0 (0.0%) | 7 (14%) | 1 (1.9%) | 11 (8.5%) | 36 (12.8%) |
pol 1: grass pollen group 1 (includes β-expansins n Lol p 1 from Lolium perenne and r Phl p 1 from Phleum pratense); pol 4,5: includes r Phl p 5 ribonuclease; n Cyn d 1: Group 1 β-expansin of Cynodon dactylon; n Pru p 3: peach lipid transfer protein; r Cor a 8: hazelnut lipid transfer protein; n Art v 3: Mugwort lipid transfer protein; r Jug r1: walnut 2S Albumin’ r Ani s 1: serin-protease inhibitor of Anisakis simplex.
n: native allergen; r: recombinant allergen.
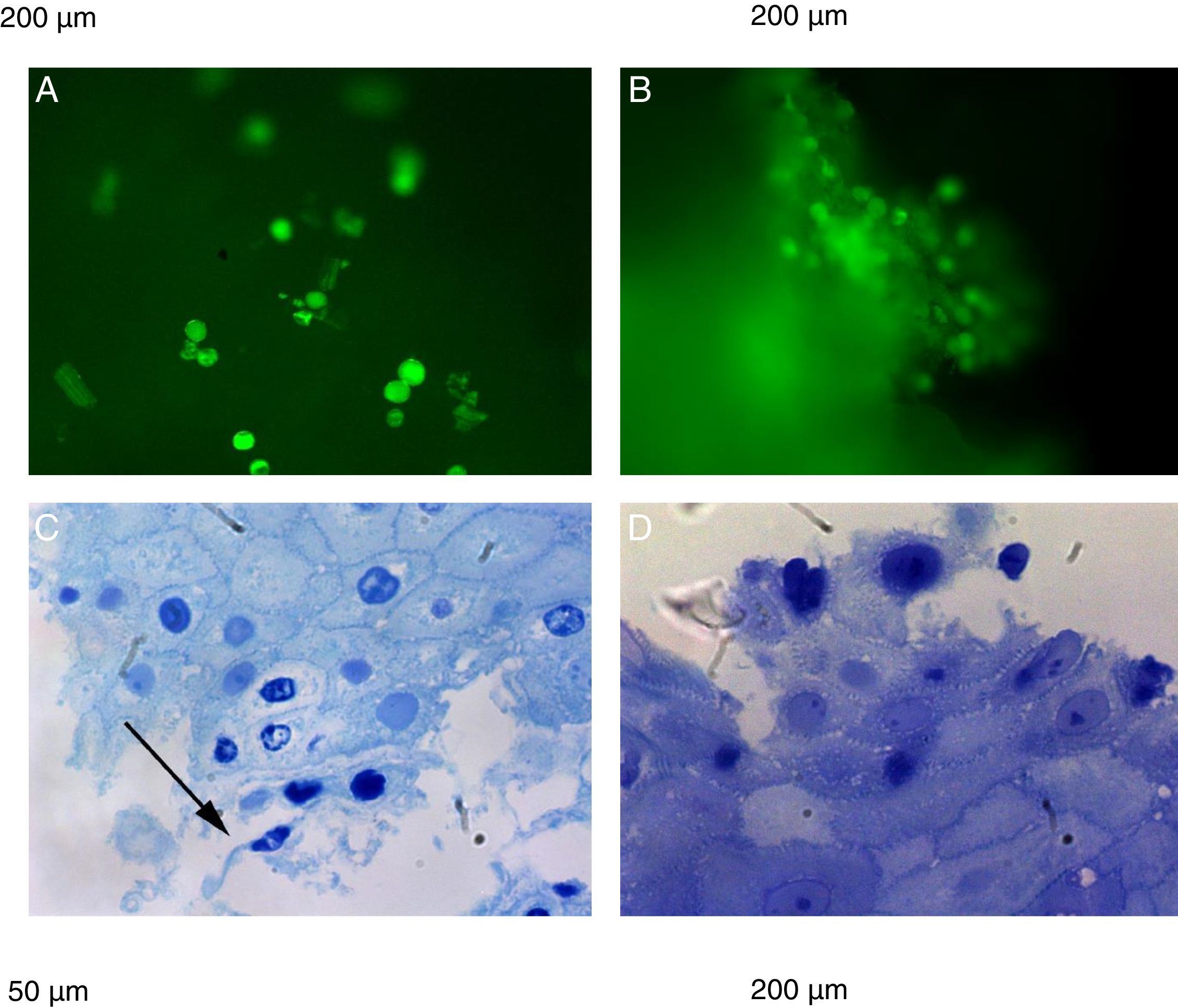
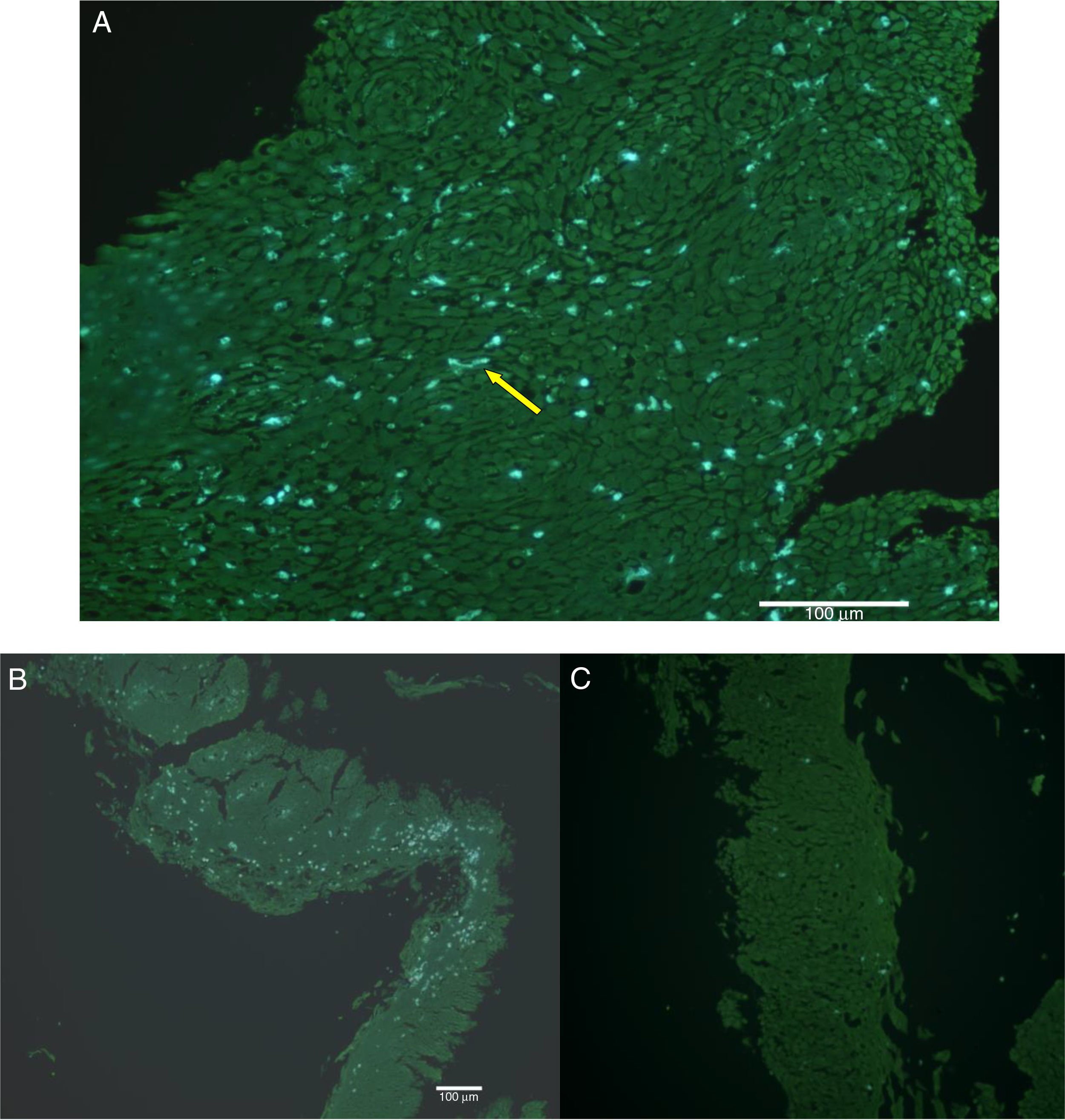
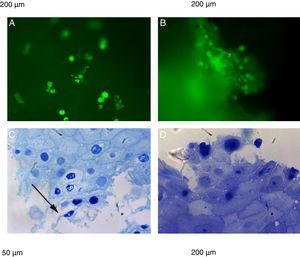
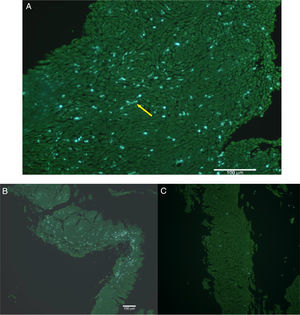
The natural fluorescence of pollen, spores and other plant elements is easily observed on the surface of esophageal biopsies under epifluorescence before histological fixation (Fig. 3). In the contact area between pollen and esophageal mucosa the epithelial cells are frequently damaged and detached from the rest of the stratum spinosum. Pollen and/or cell-penetrating pollen tubes were observed in 80 (65.6%) EoE patients with positive CRD to group 1 grass pollens (55 patients) and other pollen mixtures (25 patients) (Fig. 2).
Epifluorescence (A and B) shows pollen, spores and other plant elements on the surface of esophageal biopsies before histological fixation. (C and D) plant impactions and esophageal mucosae showing damaged spinous epithelial cells. Semi-thin sections with toluidine blue stain. Arrow shows pollen tubes.
SEM showed numerous Poaceae family dehydrated pollen grains infiltrating intercellular spaces, in biopsies obtained both in the spring and summer (Fig. 4).
Human histology showed eosinophilic infiltration before AIT and elimination diet with significant decrease of eosinophil infiltrate at two years.
EoE biopsies showed eosinophilic infiltration gradually lessened after etiological treatment with diet and specific AIT (before AIT H/E 100×, >15 Eo/CGA: after AIT (H/E 40×). H/E: hematoxylin–eosin stain).
TherapyAIT was administered in 91 EoE patients: grass pollen AIT (55 patients), AIT to other pollens/mixtures (26 patients), mites (7), alternaria (2) and epithelia (1 patient). AIT was sublingual in 61 patients (59.4%) and subcutaneous in 42 (40.6%), and grass pollen AIT was subcutaneous in 26 patients and sublingual in 29. Grass pollen AIT (subcutaneous, triticum aestivum pollen) was administered in 10 CD patients.
Comparison of EoE patients sensitized to pollens on CRD who did and did not receive AIT showed AIT-treated patients had better outcomes (odds ratio 177.3, 95% CI 16.2–1939.0). Improvement was observed in 122 (94.8%) patients with EoE, 43 (80%) with CD, and 100% with pollen asthma after two years AIT. There were no relapses in 101 (78.9%) EoE patients and 45 (85%) CD patients. CRD-directed elimination diet in EoE patients consisted of one food in 36 patients and two groups in 12 patients (mostly fruits or nuts, especially hazelnut and walnut) (Table 2).
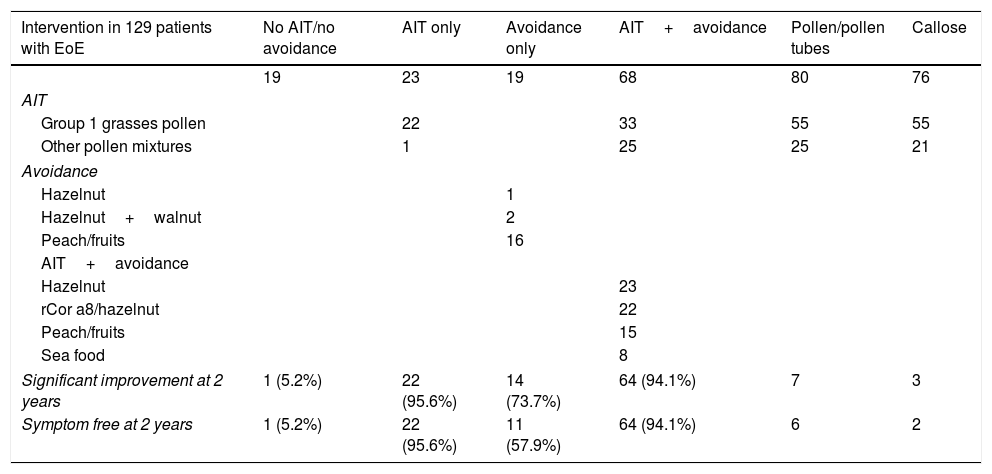
Clinical outcomes of EoE patients after AIT and/or elimination diet.
| Intervention in 129 patients with EoE | No AIT/no avoidance | AIT only | Avoidance only | AIT+avoidance | Pollen/pollen tubes | Callose |
|---|---|---|---|---|---|---|
| 19 | 23 | 19 | 68 | 80 | 76 | |
| AIT | ||||||
| Group 1 grasses pollen | 22 | 33 | 55 | 55 | ||
| Other pollen mixtures | 1 | 25 | 25 | 21 | ||
| Avoidance | ||||||
| Hazelnut | 1 | |||||
| Hazelnut+walnut | 2 | |||||
| Peach/fruits | 16 | |||||
| AIT+avoidance | ||||||
| Hazelnut | 23 | |||||
| rCor a8/hazelnut | 22 | |||||
| Peach/fruits | 15 | |||||
| Sea food | 8 | |||||
| Significant improvement at 2 years | 1 (5.2%) | 22 (95.6%) | 14 (73.7%) | 64 (94.1%) | 7 | 3 |
| Symptom free at 2 years | 1 (5.2%) | 22 (95.6%) | 11 (57.9%) | 64 (94.1%) | 6 | 2 |
After two years CRD-directed AIT and/or elimination diet, EoE patients showed significant clinical improvement (p<0.017) and 97 (75.2%) were discharged (negative biopsy, no symptoms, no medication) without relapse. Mean eosinophils fell from 130/field (range 876–20) to 3/field (range 43–0). The mean dysphagia score fell from 9 (range 10–0) to 1 after AIT in EoE patients. There were no adverse reactions to AIT.
Callose detection fell significantly in patients treated with pollen AIT (p>0.001), in tandem with the reduction in eosinophilic infiltrate and the shedding of spinous epithelial cells.
All patients were offered the reintroduction of the food eliminated in a controlled, double-blind, placebo-controlled trial but this was rejected by all patients, who did not wish to risk a relapse.
DiscussionThe results of this study show that CRD-directed AIT and/or elimination diet resulted in improvements in 75.2% of EoE patients and improvement in 83.3% of CD patients. In EoE patients, the most common allergens detected were group 1 grass pollens and common plant and animal allergens. The food allergens detected included molecules causing cross-reactivity between pollens and plant foods, such as LTPs, which are resistant to heat and digestion, followed by hazelnut and walnut 2S albumin and profilins. Only CRD detected wheat LTPs (r Tri a 14) in CD and EoE patients and cow's milk in 10 CD patients.
Grass pollens account for 18% of total annual pollen, with significant quantities of group 1 grass pollens (Lol p 1) remaining in our atmosphere from April to August (allergen/grasses ratio=2.8pg/pollen), twice as much as group 5 grass allergens (Lol p 5, ratio 1.7pg/pollen) and greater than Pl a 1 (ratio 0.2pg/pollen).22,23 Studies show a relationship between pollination of this plant under stress (34). Group 1 pollen allergens a priori are a major risk factor for persons with pollen allergy and other respiratory problems, as shown by environmental studies.24,25
Group 1 grasses, β-expansins with a molecular weight of 31–35kDa, are involved in plant cell membrane relaxation and plant growth.26 Cyn d 1 of Cynodon has antifungal functions against claviceps, the source of alkaloids (ergonovine and ergonovinine) that cause tremorgenic syndrome in cattle after Cynodon consumption.27 In general, group 1 proteins and profilins are essential for pollen tube emission and more than 90% of patients sensitized to pollens in our area responded to this group.13 They normally provoke symptoms of rhinoconjunctivitis and asthma, but many EoE patients without respiratory symptoms also responded. We hypothesized that pollen and spores ingested by mouth breathing are also attached to many fruits and vegetables and could adhere to the oropharyngeal and esophageal mucosa, which might provide a suitable environment for their allergens.
The emission of pollen tubes requires certain levels of humidity, pH and temperature in the esophageal mucosa.22,23,26 After swallowing, pollen may adhere to the mucosa: this occurs in all people. If for some reason, in patients with EoE, there was a slowing of swallowing, or an alteration of the mucosal barrier by desmoglein alteration,7 epithelial barrier defects and eosinophil extracellular trap formation, as have been described,28 or a weakness of the innate immune response in EoE patients, that time of adherence could be longer and result eosinophil build-up, forming typical eosinophil micro-abscesses.
The seasonal worsening in EoE patients might be explained if the cause were environmental allergens.29 Histologists emphasized the high porosity and separation of esophageal mucosa cells and tissues such as the tympanic membrane30 in EoE patients, which could facilitate the penetration of pollen tubes.
Clinically, we determined the epitopes to which each patient responded, to avoid greater targeted dietary avoidance (fruits were not included in the empirical 6-food diet) and more specific and accurate hyposensitization therapy.31 Targeted AIT has been used for three years, with clinical and histological controls every six months, and has shown improvement in both. No adverse reactions to AIT have been reported.
A limitation of the study is that patients came from an area with high environmental levels of grass pollens and the results might vary in other areas, with further studies being necessary. Likewise, patients on elimination diets might have inadvertently ingested some of the restricted foods, which might explain why there were three relapses, as they were following a diet without hazelnuts, a food often included in occult form in many Spanish foods.
In conclusion, after satisfying the postulates of Henle and Koch,19 we suggest the treatment of EoE with specific AIT may not be empirical. CRD-directed AIT is safe and effective in EoE patients, in whom we found a high incidence of sensitization to swallowed pollens. Significant hypersensitivity to group 1 grass pollens (expansins) with antifungal activity and LTPs associated with more severe symptoms was found. CRD-directed diets were effective. EoE patients treated with specific AIT and limited exclusion diets improved and, currently, 79.1% have been discharged, of whom 91.4% have not relapsed.
FundingThis study was partially supported by the Spanish Ministry of Science and Innovation (Grant GL2014-52555-R) and the General Direction of Public Health, Castile and Leon (SACYL) and registered in its data base as (Expt.: GRS 1058/A/104).
Conflict of interestThe authors report no conflict of interest.
This study was partially supported by the Spanish Ministry of Science and Innovation (Grant GL2014-52555-R) and the Gerencia Regional de Castilla y León (Expt.: GRS 1058/A/104). We appreciate Prof Moisés Calderon's comments in this manuscript.
We thank all doctors, nurses and technicians from Primary Care Centers and Digestive Services for their support.