The objective of this study was to estimate the incidence of Allergic Rhinitis (AR) in young adults and its risk or protective factors.
MethodsA population-based prospective cohort study was carried out in 2012. The cohort participated in the International Study of Asthma and Allergy in Childhood in Castellon in 1994 and 2002. A telephone survey was conducted using the same questionnaires. A new case of AR was defined as the participants free of the disease in 2002, who self-reported suffering from AR or taking medications for AR in the period 2002–2012.
ResultsOf the 1805 schoolchildren in the cohort in 2002, 1435 young adults (23–25 years old) participated (follow-up 79.1%) in 2012; 743 were female and 692 male; their mean age was 24.9±0.6 years. Two hundred new cases of AR occurred in 1259 participants free of the disease with an incidence of 17.3 per 1000 person–years, and the incidence increased from 2002 (RR=1.42; 95% CI 1.15–1.75). The risk factors of AR adjusted by age and gender were sinusitis (RR=1.77; 95% CI 1.16–2.68), atopic dermatitis (RR=1.51; 95% CI 1.11–2.06) and constant exposure to truck traffic (RR=1.88; 95% CI 1.12–3.17). For male participants, the risk factors were asthma, sinusitis and atopic dermatitis, and for females bronchitis was a risk factor and presence of older siblings a protective factor.
ConclusionsAn increase in AR incidence was observed. Sinusitis, atopic dermatitis and constant exposure to truck traffic were the risk factors of the AR with some differences by gender.
The prevalence of allergic rhinitis (AR) and its repercussion on the life and work of patients have been studied in Spain.1–3 In the years 2002–2008, the prevalence of AR in the adult population, adolescents (13–14 years old) and schoolchildren (6–7 years old), was estimated as 21.5%, 11.1% and 9.3%, respectively. These data were obtained following different methodologies.4–6 Others studies have also established the characteristics of Spanish adults and paediatric patients with AR.7–9 However, AR could be under-diagnosed by as much as 48%.4 While these data reflect the extent of AR in the Spanish population, few cohort studies have been conducted on the incidence of AR in the adult population and their risk factors.
The European Community Respiratory Health Study (ECRHS)10 on adults from 22 to 44 years studied the risk factors of AR for all the European participants, including five centres in Spain (Albacete, Barcelona, Galdakao, Huelva and Oviedo). The AMICS birth cohorts from Barcelona and Menorca presented results of AR in the European Birth Cohort,11 and the study by Tornador-Gaya et al. in Castellon,12 was a prospective cohort study on schoolchildren who participated in the International Study of Asthma and other Allergies in Childhood (ISAAC) in 1994 and in 2002, where only the incidence of AR by gender was indicated.
The objective of this study was therefore to the follow up the cohort of schoolchildren in Castellon from 2002 (13–15 years old) to 2012 (23–25 years old) in order to estimate the incidence of AR and its risk factors and to compare it with the cohort study of the period 1994–2002.12
Patients and methodsA prospective population-based cohort study was carried out on the cohort of young adults who had participated in the phases I (6–7 years old) and III (13–15 years old) of the ISAAC in 1994 and 2002, respectively. The study was conducted from January to June 2012, the same period as in the previous studies. The same questionnaires of previous studies were used with some additional questions. The information was obtained through telephone interviews of participants carried out by health staff of the Public Health Centre. The questionnaire included items on asthma, eczema and allergic rhinitis following the ISAAC methodology. In addition, information about AR risk factors was based on specific questionnaires completed by the parents of participants in the phases I and III of the ISAAC study. The definition of AR was based on a positive self-reported response for one at least of the following questions:
- -
Have you ever had a nasal allergy including hay fever and rhinitis?
- -
Do you take any medication for allergic rhinitis?
Of the 1805 participants in the 2002 study, 1435 (79.5%) adolescents were followed up to 2012, with a loss of 370 (20.5%) adolescents. Of the 1435 participants with follow-up data, 1259 were free of AR in 2002.
Statistical analysesThe cumulative incidence of AR was estimated considering the new cases and the rest of participants and the persons-years of follow up of the cohort during 10 years. Chi2 and Fisher tests were applied to compare qualitative variables and the Kruskal–Wallis test was conducted for quantitative variables. Poisson regression was used to obtain cumulative incidence rate of AR and relative risk (RR) with a 95% confidence interval (CI). Poisson regression models were used in the bivariate and multivariate analysis to study the relationship between AR and risk or protective factors. For the multivariate analyses, independent covariates associated with AR and an alpha value less than p<0.20 were included in the model to arrive at a model in which all covariates had a significant association. The models were then adjusted by gender and age. No interactions were observed among significant variables and all models had a goodness of fit. The Stata® programme version 12 was used in the statistical analyses.
The Castellon General Hospital ethics committee approved the study and an informed consent was obtained from all participants.
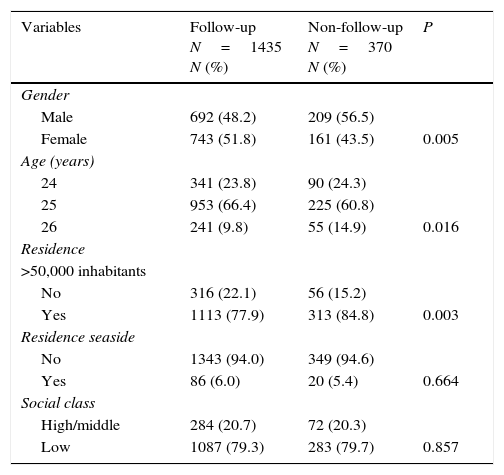
ResultsThe follow-up rate from 2002 to 2012 was 79.5% (1435/1805) with 743 females and 692 males and their mean age was 24.9±0.6 years. Some significant differences between the follow-up group and non-follow-up group are shown in Table 1; follow-up was higher among females, older age groups, and residents in cities with fewer than 50,000 inhabitants. Social class and living on the coast were not associated with the follow-up group.
Characteristic of Castellon cohort comparison of follow-up group with non-follow-up group.
| Variables | Follow-up N=1435 N (%) | Non-follow-up N=370 N (%) | P |
|---|---|---|---|
| Gender | |||
| Male | 692 (48.2) | 209 (56.5) | |
| Female | 743 (51.8) | 161 (43.5) | 0.005 |
| Age (years) | |||
| 24 | 341 (23.8) | 90 (24.3) | |
| 25 | 953 (66.4) | 225 (60.8) | |
| 26 | 241 (9.8) | 55 (14.9) | 0.016 |
| Residence | |||
| >50,000 inhabitants | |||
| No | 316 (22.1) | 56 (15.2) | |
| Yes | 1113 (77.9) | 313 (84.8) | 0.003 |
| Residence seaside | |||
| No | 1343 (94.0) | 349 (94.6) | |
| Yes | 86 (6.0) | 20 (5.4) | 0.664 |
| Social class | |||
| High/middle | 284 (20.7) | 72 (20.3) | |
| Low | 1087 (79.3) | 283 (79.7) | 0.857 |
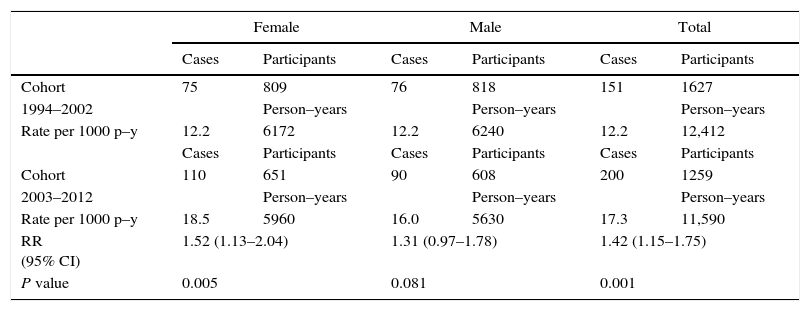
The 1994–2002 and 2003–2012 periods are compared in Table 2. The cumulative incidence per 1000 person–years of the total increases from 12.2 (151 new cases/12,412 person–years) in the first period to 17.3 (200 new cases/11,590 person–years) in the second with significant differences (RR=1.42; 95% CI 1.15–1.75). When comparing by gender, significant increases for female but not for male participants were observed (RR=1.52; 95% CI 1.13–2.04) and (RR=1.31; 95% CI 0.97–1.78), respectively.
Incidence of allergic rhinitis by gender and age in Castellon cohorts 1994–2002 and 2003–2012 with 8 and 10 years of follow up respectively.
| Female | Male | Total | ||||
|---|---|---|---|---|---|---|
| Cases | Participants | Cases | Participants | Cases | Participants | |
| Cohort | 75 | 809 | 76 | 818 | 151 | 1627 |
| 1994–2002 | Person–years | Person–years | Person–years | |||
| Rate per 1000 p–y | 12.2 | 6172 | 12.2 | 6240 | 12.2 | 12,412 |
| Cases | Participants | Cases | Participants | Cases | Participants | |
| Cohort | 110 | 651 | 90 | 608 | 200 | 1259 |
| 2003–2012 | Person–years | Person–years | Person–years | |||
| Rate per 1000 p–y | 18.5 | 5960 | 16.0 | 5630 | 17.3 | 11,590 |
| RR (95% CI) | 1.52 (1.13–2.04) | 1.31 (0.97–1.78) | 1.42 (1.15–1.75) | |||
| P value | 0.005 | 0.081 | 0.001 | |||
p–y, person–years; RR, relative risk; CI, confidence interval.
Person–years calculations=(Participants without allergic rhinitis×years)+(New cases allergic rhinitis×years/2).
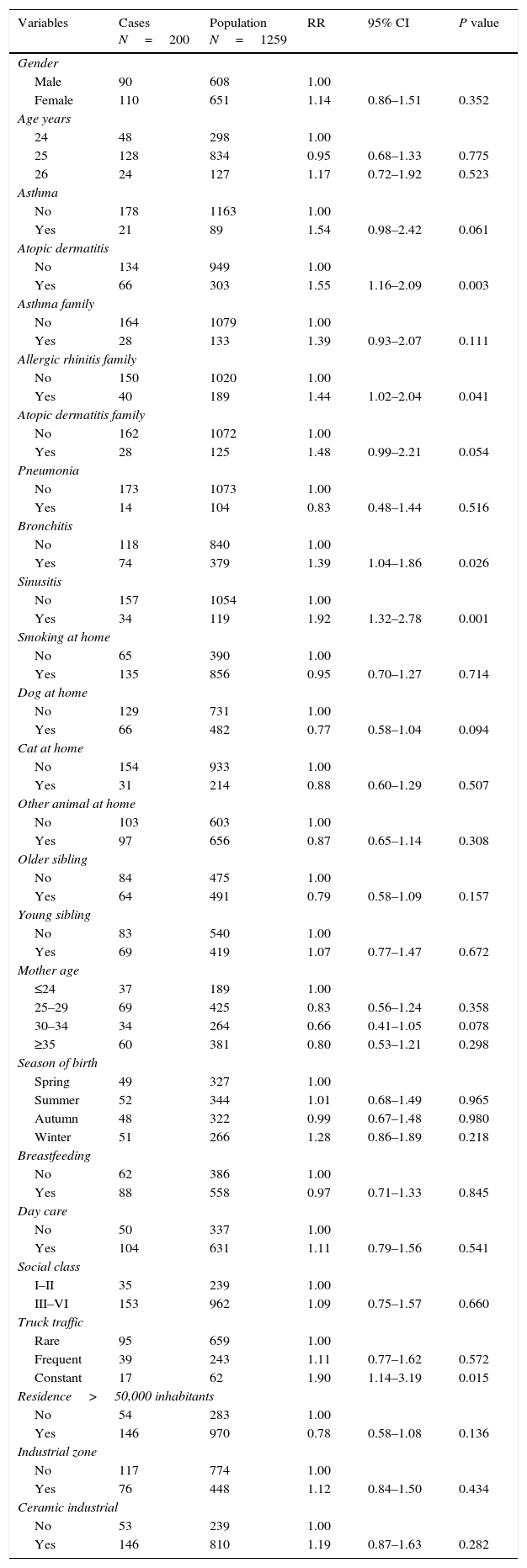
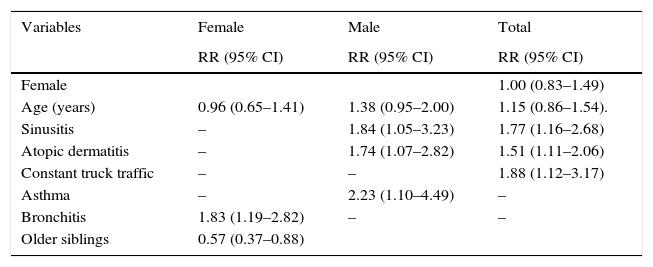
The bivariate analysis is presented in Table 3. The AR incidence was higher in female than male participants although the difference was not significant (RR=1.14; 95% CI 0.86–1.51). Some risk factors significantly associated with AR include family history of AR, history of atopic dermatitis, history of bronchitis, history of sinusitis, and constant exposure to truck traffic. In the multivariate analysis (Table 4), three risk factors were associated with the incidence of AR adjusted by age and gender (Table 4), sinusitis (RR=1.77; 95% CI 1.16–2.68) and atopic dermatitis (RR=1.51; 95% CI 1.11–2.06) and constant exposure to truck traffic (RR=1.88; 95% CI 1.12–3.17). For males, risk factors were asthma (RR=2.23; 95% CI 1.10–4.49), sinusitis (RR=1.84 95% CI 1.05–3.23) and atopic dermatitis (RR=1.74; 95% CI 1.07–2.82) and for females, a risk factor, bronchitis (RR=1.83; 95% CI 1.19–2.82) and a protective factor was the presence of older siblings (RR=0.57; 95% CI 0.37–0.88).
Bivariate analysis of risk factors of allergic rhinitis Castellon cohort by Poisson regression.
| Variables | Cases N=200 | Population N=1259 | RR | 95% CI | P value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 90 | 608 | 1.00 | ||
| Female | 110 | 651 | 1.14 | 0.86–1.51 | 0.352 |
| Age years | |||||
| 24 | 48 | 298 | 1.00 | ||
| 25 | 128 | 834 | 0.95 | 0.68–1.33 | 0.775 |
| 26 | 24 | 127 | 1.17 | 0.72–1.92 | 0.523 |
| Asthma | |||||
| No | 178 | 1163 | 1.00 | ||
| Yes | 21 | 89 | 1.54 | 0.98–2.42 | 0.061 |
| Atopic dermatitis | |||||
| No | 134 | 949 | 1.00 | ||
| Yes | 66 | 303 | 1.55 | 1.16–2.09 | 0.003 |
| Asthma family | |||||
| No | 164 | 1079 | 1.00 | ||
| Yes | 28 | 133 | 1.39 | 0.93–2.07 | 0.111 |
| Allergic rhinitis family | |||||
| No | 150 | 1020 | 1.00 | ||
| Yes | 40 | 189 | 1.44 | 1.02–2.04 | 0.041 |
| Atopic dermatitis family | |||||
| No | 162 | 1072 | 1.00 | ||
| Yes | 28 | 125 | 1.48 | 0.99–2.21 | 0.054 |
| Pneumonia | |||||
| No | 173 | 1073 | 1.00 | ||
| Yes | 14 | 104 | 0.83 | 0.48–1.44 | 0.516 |
| Bronchitis | |||||
| No | 118 | 840 | 1.00 | ||
| Yes | 74 | 379 | 1.39 | 1.04–1.86 | 0.026 |
| Sinusitis | |||||
| No | 157 | 1054 | 1.00 | ||
| Yes | 34 | 119 | 1.92 | 1.32–2.78 | 0.001 |
| Smoking at home | |||||
| No | 65 | 390 | 1.00 | ||
| Yes | 135 | 856 | 0.95 | 0.70–1.27 | 0.714 |
| Dog at home | |||||
| No | 129 | 731 | 1.00 | ||
| Yes | 66 | 482 | 0.77 | 0.58–1.04 | 0.094 |
| Cat at home | |||||
| No | 154 | 933 | 1.00 | ||
| Yes | 31 | 214 | 0.88 | 0.60–1.29 | 0.507 |
| Other animal at home | |||||
| No | 103 | 603 | 1.00 | ||
| Yes | 97 | 656 | 0.87 | 0.65–1.14 | 0.308 |
| Older sibling | |||||
| No | 84 | 475 | 1.00 | ||
| Yes | 64 | 491 | 0.79 | 0.58–1.09 | 0.157 |
| Young sibling | |||||
| No | 83 | 540 | 1.00 | ||
| Yes | 69 | 419 | 1.07 | 0.77–1.47 | 0.672 |
| Mother age | |||||
| ≤24 | 37 | 189 | 1.00 | ||
| 25–29 | 69 | 425 | 0.83 | 0.56–1.24 | 0.358 |
| 30–34 | 34 | 264 | 0.66 | 0.41–1.05 | 0.078 |
| ≥35 | 60 | 381 | 0.80 | 0.53–1.21 | 0.298 |
| Season of birth | |||||
| Spring | 49 | 327 | 1.00 | ||
| Summer | 52 | 344 | 1.01 | 0.68–1.49 | 0.965 |
| Autumn | 48 | 322 | 0.99 | 0.67–1.48 | 0.980 |
| Winter | 51 | 266 | 1.28 | 0.86–1.89 | 0.218 |
| Breastfeeding | |||||
| No | 62 | 386 | 1.00 | ||
| Yes | 88 | 558 | 0.97 | 0.71–1.33 | 0.845 |
| Day care | |||||
| No | 50 | 337 | 1.00 | ||
| Yes | 104 | 631 | 1.11 | 0.79–1.56 | 0.541 |
| Social class | |||||
| I–II | 35 | 239 | 1.00 | ||
| III–VI | 153 | 962 | 1.09 | 0.75–1.57 | 0.660 |
| Truck traffic | |||||
| Rare | 95 | 659 | 1.00 | ||
| Frequent | 39 | 243 | 1.11 | 0.77–1.62 | 0.572 |
| Constant | 17 | 62 | 1.90 | 1.14–3.19 | 0.015 |
| Residence>50,000 inhabitants | |||||
| No | 54 | 283 | 1.00 | ||
| Yes | 146 | 970 | 0.78 | 0.58–1.08 | 0.136 |
| Industrial zone | |||||
| No | 117 | 774 | 1.00 | ||
| Yes | 76 | 448 | 1.12 | 0.84–1.50 | 0.434 |
| Ceramic industrial | |||||
| No | 53 | 239 | 1.00 | ||
| Yes | 146 | 810 | 1.19 | 0.87–1.63 | 0.282 |
RR, relative risk; CI, confidence interval.
Some items had incomplete information.
Associated risk and protective factors of Castellon cohort by Poisson regression analysis.
| Variables | Female | Male | Total |
|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Female | 1.00 (0.83–1.49) | ||
| Age (years) | 0.96 (0.65–1.41) | 1.38 (0.95–2.00) | 1.15 (0.86–1.54). |
| Sinusitis | – | 1.84 (1.05–3.23) | 1.77 (1.16–2.68) |
| Atopic dermatitis | – | 1.74 (1.07–2.82) | 1.51 (1.11–2.06) |
| Constant truck traffic | – | – | 1.88 (1.12–3.17) |
| Asthma | – | 2.23 (1.10–4.49) | – |
| Bronchitis | 1.83 (1.19–2.82) | – | – |
| Older siblings | 0.57 (0.37–0.88) |
In the area of Castellon the incidence of AR between 13–15 years old and 23–25 years old was 17.3 per 1000 person–years and it was associated with respiratory infections (sinusitis and bronchitis), allergic diseases (atopic dermatitis, asthma), and pollution (constant exposure to truck traffic); to having older siblings was a protective factor.
A significant increase of AR from the 1994–2002 Castellon cohort study was observed12 with an incidence rate of AR of 12.2 per 1000 persons/year, and 17.3 per 1000 persons/year in the present study, although the follow-up of the two studies were 8 and 10 years, respectively. By gender, a higher and significant increase was found for female participants. The increase of AR with age and in higher rates for females have been observed in some studies but it is not general.10,13–15 However, comparisons are difficult to make when case definition, periods of study, and AR diagnosis are taken into account. The incidence of our study is therefore located mid-way between cohort studies of 8% and 11% in German adolescents13,16 and 21% in Swedish children between 8 and 16 years.17 Incidences of AR in a cohort of Finnish adolescent to young adults (age 16–32) was 13.4 per 1000 person–years in males and 11.4 per 1000 person–years in females18 and in a German cohort (age 3–20) 22 per 1000 person–years.19
The AR risk factors in the 1994–2002 cohort study were family history of AR (RR=1.88; 95% CI 1.06), high social class (groups I–II versus III–VI) (RR=2.08; 95% CI 1.64–3.23), mother's age above 34 years (RR=2.35; 95% CI 1.07–5.08), rhinitis in the last 12 months (RR=2.04; 95% CI 1.03–4.06) and being born between October and March (RR=1.62; 95% CI 1.04–2.59). In the present study, some of these risk factors, such as family history of AR, are significantly associated with AR in the bivariate analysis, but not in the multivariate analysis, suggesting that risk factors may change as adolescents developed.
In our study the risk factors were history of sinusitis and history of atopic dermatitis for all the participants and constant exposure to truck traffic. In the study of prevalence with this cohort in 2002,20 some of these factors were associated with AR, such as history of sinusitis, bronchitis, and the protective factor was the presence of an older sibling. Some of these factors are found in other cohort studies, including history of eczema,19,21 history of bronchitis and history of sinusitis,22 traffic exposure,23 and presence of older sibling.24 In addition, the multi-morbidity of AR, asthma and eczema have been indicated.25 However, in cohort studies of traffic-related air pollution and birth order, gene–environment interactions were not demonstrated.26,27
By gender, history of asthma, sinusitis and atopic dermatitis were risk factors for male participants, whereas for female participants, history of bronchitis was a risk factors and the presence of an older sibling was a factor of protection. In the Isle of Wight cohort, Kurukulaaratchy et al.28 have found non-atopic rhinitis prevalence to be higher in females than in males, and the contrary for atopic rhinitis. The risk factors differences between females and males in our study are in line with the findings of these authors. A German cohort study 16 found two AR protection factors for girls from 9–11 years old to 19–24 years old, namely late onset of menarche and use of hormonal contraceptives.
Altogether, the associations between AR and the risk factors were modest considering the epidemiological design with RR less than 3.0.29 The estimated attributable fractions30 for sinusitis, atopic dermatitis, and constant exposure to truck traffic in the cohort were 7.7%, 11.2% and 5.3%, respectively. However, all these factors represent only 24.2% of the AR risk. Other risk AR factors identified in prospective cohort studies such as Kelberger et al.,13 in Munich and Dresden with 2557 participants from 9–11 years to 15–18 years were parental history of asthma, positive skin prick test response to allergens, female sex, and no exclusive breast-feeding or for less than two months. The probability of AR incidence with all these factors reached 72%. However, the case definition of AR included physician-diagnosis of AR and current symptoms in the last 12 months. In addition, in the cohort of ECRHS of adults from 20 to 44 years and follow-up a mean of 8.9 years, Matheson et al.10 found that females and parental allergy were risk factors for the incidence of AR, and presence of siblings and exposure to dogs before the age of five were protective factors.
The results for the risk factors suggest than the genesis of the AR is an intricate process with many influencing factors, which could vary from childhood to young adulthood in a context of gene–environment interactions.31 On the basis of these considerations, the cohort design is an appropriate method to investigate allergy and asthma and the European birth cohorts are a good example.32,33
The study has some strength, including its prospective design, sufficient sample size to detect potential risk factors, the acceptable follow-up, and the same core questionnaire. However, it also has the following limitations: the follow-up loses were associated with gender, age and residence in populations >50,000 inhabitants, some items had incomplete information, some AR risk factors, such as diet and home characteristics were not included in the questionnaire,34–36 no objective tests were used and the case definition of AR was based on self-reported AR. It was not possible to distinguish between non-atopic and atopic rhinitis, and the ISAAC questionnaire did not include the new approach to AR from ARIA37 (Allergic Rhinitis and its Impact on Asthma) and local AR entity.38
ConclusionA significant increase of the AR incidence was observed in young adults. This incidence is situated at a mid-point when compared with other young adult cohorts. History of sinusitis, atopic dermatitis and constant exposure to truck traffic were the risk factors of the AR with some differences by gender.
Conflict of interestThe authors have no conflict of interest to declare.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
We thank the participants and their families for their cooperation in making the study possible.