Although it is well known that allergic diseases involve a strong Th2 immune response, with production of high levels of specific IgE allergen, knowledge on the association between filarial infection and allergies, among paediatric patients is scarce.
ObjectiveTo evaluate the allergic response patterns in cases of filarial infection by comparing peripheral eosinophils, total IgE levels, immediate hypersensitivity and cytokine levels in children and adolescents in Brazil.
MethodsThis was an exploratory study with three groups: (I) with filarial infection and without allergic diseases; (II) without filarial infection and with allergic diseases; and (III) without filarial infection and without allergic diseases. The prick test and specific IgE tests for aeroallergens were performed using five antigens. Peripheral eosinophils and total IgE were also evaluated. IL-4 and IL-5 were determined using whole-blood culture stimulated by three antigens.
ResultsEosinophilia and elevated levels of total IgE (≥400IU/dl) were observed in all groups. The prick test was positive in 56.6% of the cases. Group I presented hypersensitive responses similar to the allergic disease groups. In the whole-blood culture stimulated by Dermatophagoides pteronyssinus, average IL-4 production did not differ significantly among the groups, but IL5 production resulting from stimulation was greater in the allergic disease groups (p<0.05).
ConclusionsThe allergic response pattern in group with filarial infection was similar to that of the groups with and without allergic diseases, but the response to IL-5 in the culture stimulated by D. pteronyssinus was an exclusive characteristic of the allergic group.
Lymphatic filariasis is caused by the nematodes Wuchereria bancrofti, Brugia timori or Brugia malayi.1 It evolves as a chronic disease, particularly due to its Th2-type immune response. This response includes increased production of IL-4, IL-5 and IL-3, along with presence of eosinophilia and IgE production.2 These characteristics are quite similar to those of schistosomiasis.3
Although it is well known that allergic diseases involve a strong Th2 immune response, with production of high levels of specific IgE allergen, knowledge on the association between filarial infection by W. bancrofti and allergies is scarce, particularly in relation to paediatric patients.4,5 During the course of filarial infection, in which the disease may become chronic, the host's immune response seems to be modulated towards a regulatory profile through regulatory T cells that produce IL-10 and TGF-β, and through production of IgG4 antibodies via B cells. This process makes the host tolerant to the parasite, thus decreasing the tissue damage caused by an excessive inflammatory immune response.6,7
It needs to be considered that a similar phenomenon might also regulate the allergic response. In this regard, individuals infected by Schistosoma mansoni and by Trichuris trichiura may present low incidence of allergies.8–10 Thus, the interaction between helminth infections and allergic disease remains unclear.8–11 Divergences in opinions regarding this interaction may be due to several factors, such as the parasite species, the parasite load, the time elapsed since infection and the host's age, immune response or genetics.6
In addition, some authors have reported the existence of cross-reactivity between helminth antigens and the usual allergens.6,12,13 This cross-reactivity may involve epitopes of antibodies produced by B cells.2,6 Strong antigenic similarity between tropomyosins from Onchocerca volvulus and from Dermatophagoides pteronyssinus (Dpt 10), which are associated with increased IgE and IgG4 antibody production in subjects with chronic filarial infection, has been demonstrated.6
A bibliographical survey identified gaps in knowledge regarding this relationship between filariasis and allergy, particularly in children, and this motivated the present investigation. Research on the association between skin response to allergens and lymphatic filarial infection will provide support for the Global Programme to Eliminate Lymphatic Filariasis, with regard to understanding the frequency of occurrences of allergic diseases in populations after mass treatment.14 We evaluated the allergic response using cases of filarial infection as a model, through analysis on peripheral eosinophils, total IgE levels, immediate hypersensitivity reactions and IL-4 and IL-5 cytokine levels in children and adolescents, in an area in Brazil that is endemic for lymphatic filariasis.
MethodsThis study was conducted among individuals living in the municipalities of Recife, Olinda and Jaboatão dos Guararapes, in Brazil, where lymphatic filariasis is endemic.15 These municipalities present similar socioeconomic conditions characterised by precarious conditions of sanitation, sewage disposal, wastewater management and water supply. Such conditions favour the creation of artificial breeding sites for vectors. Environmental factors interact strongly with socioeconomic factors, thereby contributing towards maintaining the endemicity of lymphatic filariasis.16 These attributes relate to socioeconomic conditions and to the characteristics of the quality of the urban infrastructure and environment that affect health and contribute to other health problems such as intestinal parasites.17
This was an exploratory study involving children and adolescents younger than 16 years of age. The sample size calculation was based on the frequency of positivity in skin tests for aeroallergens found in asthmatic and asymptomatic patients according to Godinho et al.18 The sample size thus calculated was sufficient to detect differences between the groups, according to the estimates made using the STATA 9.1 software. To compare proportions between the groups regarding the results from skin tests for aeroallergens, the following assumptions were made: proportions of 15% positivity in the non-asthmatic group and 80% positivity in the asthmatic group; alpha value=0.05 (two-tailed); power=90%; and group I to group II ratio=1. From these assumptions, the sample size required in each group would be 14 individuals. In the end, a sample composed of 20 participants in each group was studied, i.e. a total of 60 individuals.
The exclusion criteria comprised use of diethylcarbamazine (in the last 24 months), corticosteroids (in the last 30 days) or antihistamines (in the last 10 days). Subjects harbouring intestinal parasites (A. lumbricoides, Ancylostomatidae, T. trichiura, S. mansoni or Strongyloides stercoralis) were also excluded.
Initially, screening tests were performed to allocate the subjects to one of the three study groups:
Group I – with filarial infection and without allergic diseases (WFNA).
Group II – without filarial infection and with allergic diseases (NFWA).
Group III – without filarial infection and without allergic diseases (NFNA).
The tests consisted of: (1) diagnosis of intestinal parasites using the Hoffmann, Pons and Janer method, with three stool samples collected on different days; (2) investigation of filarial infection using three techniques, i.e. membrane filtration from a venous blood sample collected between 23:00 and 01:00h; antigen testing by means of rapid immunochromatography (ICT Binax, NOW) and the enzyme-linked immunosorbent assay (ELISA) (Og4C3)1; and determination of the presence of adult W. bancrofti worms using ultrasonography19; and (3) identification of allergic disease through the definitions of the clinical criteria for asthma (Global Initiative for Asthma, 2016), allergic rhinitis20 and atopic dermatitis,21 based on a standard validated questionnaire in accordance with the guidelines for each allergy. Individuals with allergic disease who reported having had symptoms within the preceding 12 months were included in the study.
The screening tests were performed on volunteers from an area that is endemic for lymphatic filariasis and soil-transmitted helminthiasis.17,22 Filarial infection was defined as the presence of microfilariae, while individuals without this disease were defined as those whose results from the four performed previously were negative. The children and adolescents thus identified were allocated as follows: group I (WFNA), n=20; group II (NFWA), n=20; and group III (NFNA), n=20.
As soon as the subjects had been selected, they underwent immediate hypersensitivity testing, total serum IgE testing, eosinophil counts, and an evaluation of the Th2 cellular immune response (IL-4 and IL-5).
Skin prick tests or specific IgE tests were used to evaluate immediate hypersensitivity using antigenic extracts produced by FDA Allergenic. These extracts were from dust mites (Blomia tropicalis, Dermatophagoides farinae and Dermatophagoides pteronyssinus), fungi (Aspergillus fumigatus and Penicillium notatum (var. chrysogenum)), domesticated animals’ hair (cats and dogs) and insects (Blattella germanica and Periplaneta americana). At this point, 1% histamine and a saline solution were used as positive and negative controls, respectively. A test was considered positive when the wheal diameter was greater than or equal to 3mm, relative to the control.23 A specific serum IgE assay was used as a replacement for the skin prick test for seven individuals in the WFNA group. The UniCAP system immunoassay enzyme method (Thermo Fisher) was used for this assay, and specific IgE levels ≥0.35IU/dl were considered to reflect positive results.24
For the total serum IgE test, the Immuno-CAP method (Thermo Fisher) was used, with a cut-off value ≥400IU/dl.25 Peripheral eosinophil counts were performed using the direct method, and values ≥500eos/mm3 were considered representative of eosinophilia.26
To evaluate the Th2 immune response, total blood (5ml) with heparin and without separation of the mononuclear layer was used.27 This was cultured in RPMI-1640 medium containing penicillin (100U/ml) and streptomycin (100μg/ml). The culture was stimulated using a pure Dermatophagoides pteronyssinus extract (Dpt, 10μg/ml), PMA/Iono (phorbol myristate acetate, 50ng/ml, and ionomycin, 1μg/ml), and PPD (purified protein derivative, 1mg/ml). A non-stimulated culture was used as a negative control. Next, the samples were incubated at 37°C in an incubator with 5% CO2 in a humid atmosphere. The supernatants were collected after 120h (determined after a kinetic test) and were frozen at −70°C in order to evaluate the IL-4 and IL-5 cytokine levels by means of ELISA, using a Quantikine human immunoassay kit (R&D Systems).
The data were added in pairs to the EpiInfo programme, version 7, and analyses were performed using descriptive statistics. The Kolmogorov–Smirnov test was used. To compare average values among the groups, for IL-4 and IL-5 levels and other quantitative variables, we used the Kruskal–Wallis test followed by the Mann–Whitney test for paired comparisons. Fisher's exact test and the χ2 test were used for comparisons between qualitative variables. A 5% significance level was used for all tests and comparisons were considered significant if p<0.05.
Ethical noteThis project was approved by the Research Ethics Committee of the Centro de Pesquisas Aggeu Magalhães/Fundação Oswaldo Cruz (No. 0114.0.095.000-08). We obtained a signed informed consent statement from all subjects, and those diagnosed with filarial infection or intestinal parasites received the appropriate treatment.
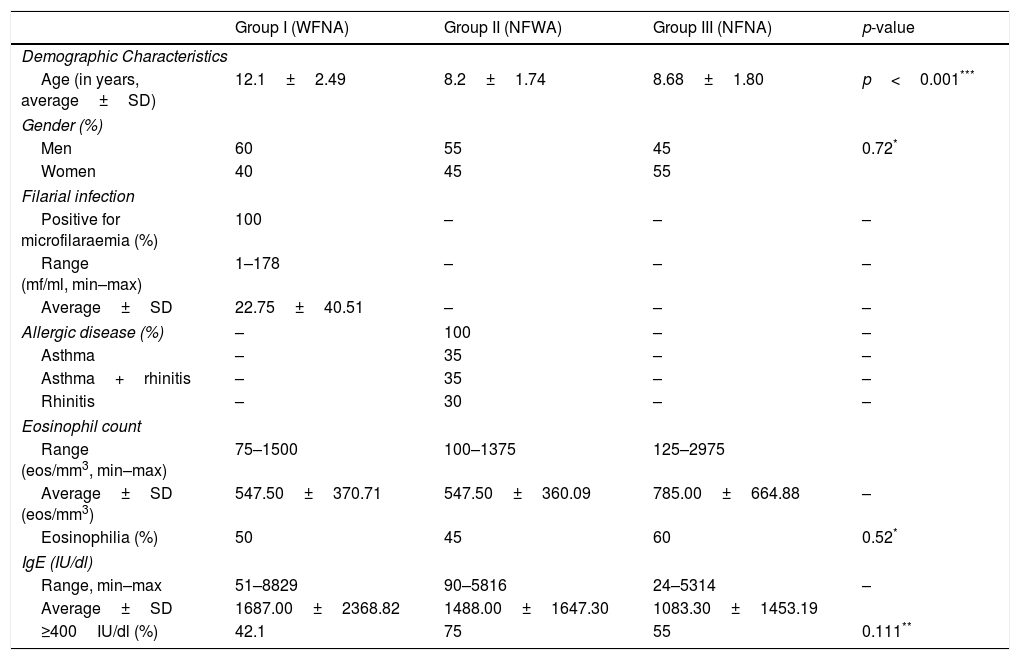
ResultsAmong the children and adolescents studied, there were slightly more male participants. The subjects’ ages ranged from 4 to 15 years, with an average of 9.7 years. The average age was highest in the WFNA group (p<0.001) (Table 1).
Demographic characteristics of tested children and adolescents from an endemic area of lymphatic filariasis area of lymphatic filariasis in Brazil, with comparative data between peripheral eosinophil counts and total IgE levels. According to their respective filarial infection status and allergic disease status, the volunteers are distributed into the following groups: filarial infection and without allergic disease (Group I, WFNA); without filarial infection and with allergic disease (Group II, NFWA) or without filarial infection and without allergic disease (Group III, NFNA).
| Group I (WFNA) | Group II (NFWA) | Group III (NFNA) | p-value | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Age (in years, average±SD) | 12.1±2.49 | 8.2±1.74 | 8.68±1.80 | p<0.001*** |
| Gender (%) | ||||
| Men | 60 | 55 | 45 | 0.72* |
| Women | 40 | 45 | 55 | |
| Filarial infection | ||||
| Positive for microfilaraemia (%) | 100 | – | – | – |
| Range (mf/ml, min–max) | 1–178 | – | – | – |
| Average±SD | 22.75±40.51 | – | – | – |
| Allergic disease (%) | – | 100 | – | – |
| Asthma | – | 35 | – | – |
| Asthma+rhinitis | – | 35 | – | – |
| Rhinitis | – | 30 | – | – |
| Eosinophil count | ||||
| Range (eos/mm3, min–max) | 75–1500 | 100–1375 | 125–2975 | |
| Average±SD (eos/mm3) | 547.50±370.71 | 547.50±360.09 | 785.00±664.88 | – |
| Eosinophilia (%) | 50 | 45 | 60 | 0.52* |
| IgE (IU/dl) | ||||
| Range, min–max | 51–8829 | 90–5816 | 24–5314 | – |
| Average±SD | 1687.00±2368.82 | 1488.00±1647.30 | 1083.30±1453.19 | |
| ≥400IU/dl (%) | 42.1 | 75 | 55 | 0.111** |
In the WFNA group, the microfilaremia ranged from 1 to 178mf/ml (with an average of 22.75mf/ml). In the NFWA group, 35% (7/20) of the subjects were identified as asthmatic, 35% (7/20) were asthmatic associated with rhinitis and 30% (6/20) were found to have allergic rhinitis. No cases were identified as atopic dermatitis (Table 1).
All three groups presented average eosinophil counts above 500eosinophils/mm3 (p>0.05). The proportions of individuals with eosinophilia were 50%, 45% and 60% in the WFNA, NFWA and NFNA groups, respectively (p=0.52).
Table 1 outlines the results regarding total serum IgE levels. It was found that 57% of the individuals presented levels above 400IU/dl, i.e. 42.1%, 75% and 55% in the WFNA, NFWA and NFNA groups, respectively (p=0.11).
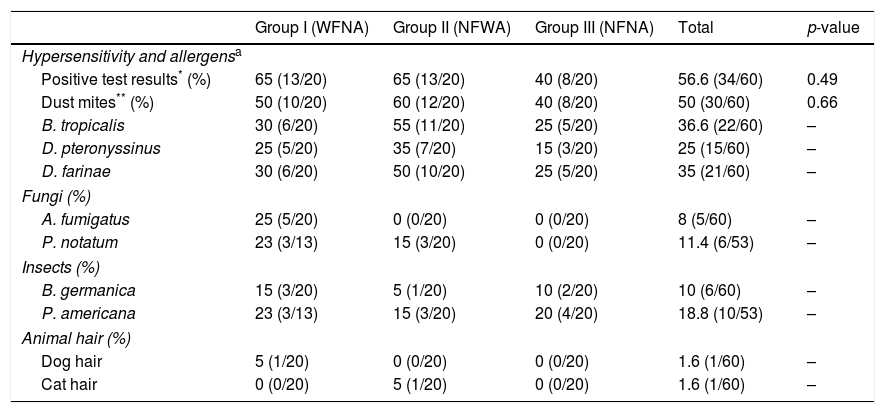
The immediate hypersensitivity test was positive in 56.6% of the cases (34/60). This was found in 65% of the WFNA and NFWA groups (13/20) and 40% of the NFNA group (p=0.49). In all groups, the highest percentage of positive hypersensitivity was to dust mites (B. tropicalis, D. pteronyssinus, and D. farinae). The dog and cat hair allergens presented the lowest percentage, with only two individuals testing positive (Table 2).
Comparative data on hypersensitivity and airborne allergens among children and adolescents from an area where lymphatic filariasis is endemic, Brazil.
| Group I (WFNA) | Group II (NFWA) | Group III (NFNA) | Total | p-value | |
|---|---|---|---|---|---|
| Hypersensitivity and allergensa | |||||
| Positive test results* (%) | 65 (13/20) | 65 (13/20) | 40 (8/20) | 56.6 (34/60) | 0.49 |
| Dust mites** (%) | 50 (10/20) | 60 (12/20) | 40 (8/20) | 50 (30/60) | 0.66 |
| B. tropicalis | 30 (6/20) | 55 (11/20) | 25 (5/20) | 36.6 (22/60) | – |
| D. pteronyssinus | 25 (5/20) | 35 (7/20) | 15 (3/20) | 25 (15/60) | – |
| D. farinae | 30 (6/20) | 50 (10/20) | 25 (5/20) | 35 (21/60) | – |
| Fungi (%) | |||||
| A. fumigatus | 25 (5/20) | 0 (0/20) | 0 (0/20) | 8 (5/60) | – |
| P. notatum | 23 (3/13) | 15 (3/20) | 0 (0/20) | 11.4 (6/53) | – |
| Insects (%) | |||||
| B. germanica | 15 (3/20) | 5 (1/20) | 10 (2/20) | 10 (6/60) | – |
| P. americana | 23 (3/13) | 15 (3/20) | 20 (4/20) | 18.8 (10/53) | – |
| Animal hair (%) | |||||
| Dog hair | 5 (1/20) | 0 (0/20) | 0 (0/20) | 1.6 (1/60) | – |
| Cat hair | 0 (0/20) | 5 (1/20) | 0 (0/20) | 1.6 (1/60) | – |
Hypersensitivity and allergens:
WNFA Group – 13 individuals received a skin prick test for all allergens.
7 individuals received the specific IgE test for all dust mite allergens, as well as for A. fumigatus, B. germanica, and for dog and cat hair.
NFWA Group received a skin prick test for all allergens.
NFNA Group received a skin prick test for all allergens.
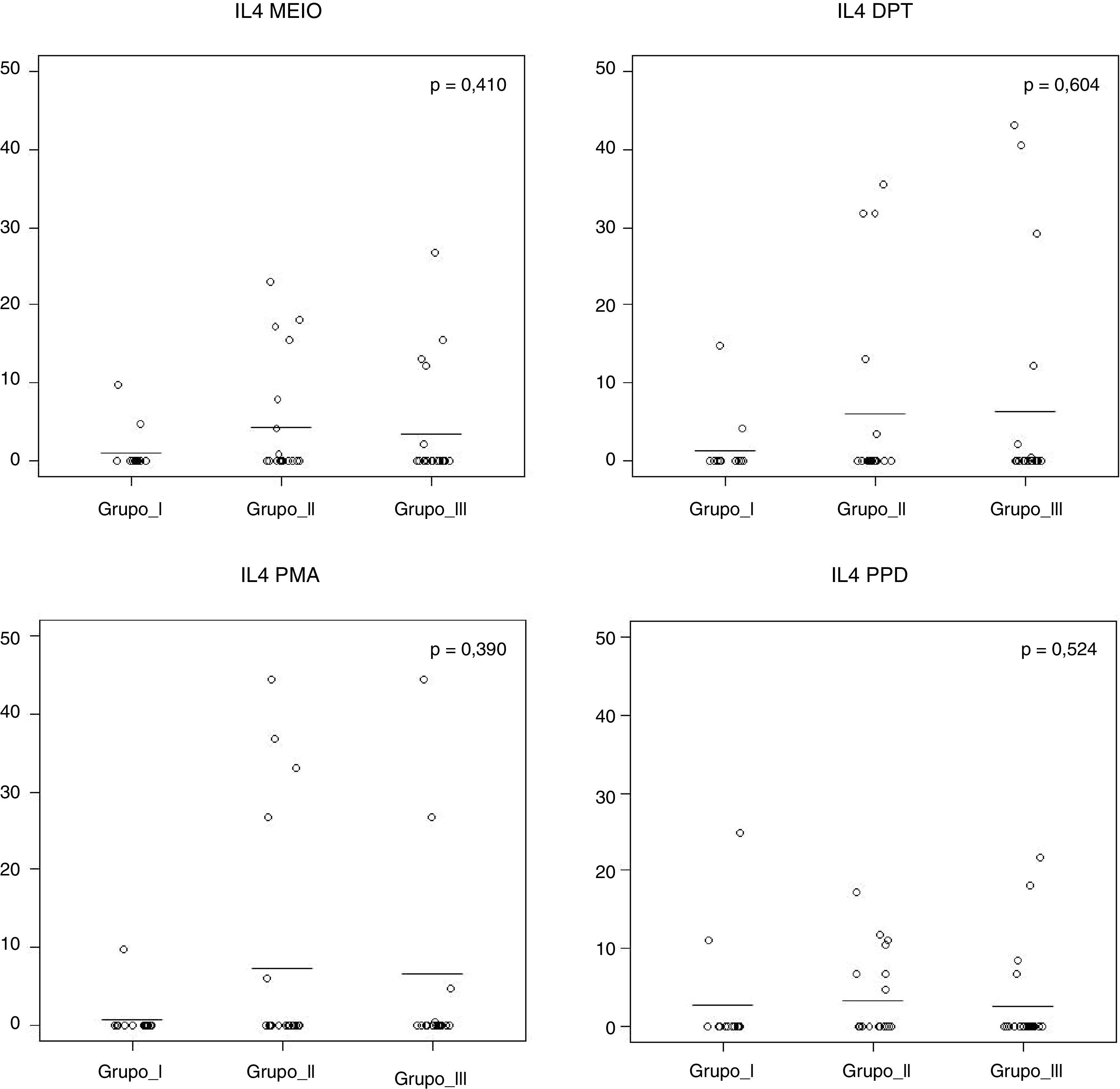
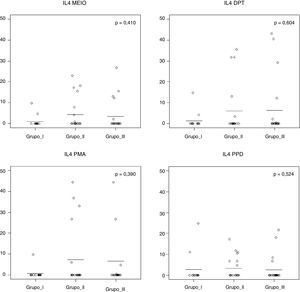
Fig. 1 shows that the IL-4 levels after blood stimulation with Dpt were similar among the three groups (p=0.60).
IL-4 levels after 120h of cell culture with stimulation from Dermatophagoides pteronyssinus spt in the three groups studied: Group I (WFNA): with filarial infection and without allergic diseases; Group II (NFWA): without filarial infection and with allergic diseases; Group III (NFNA) – without filarial infection and without allergic diseases.
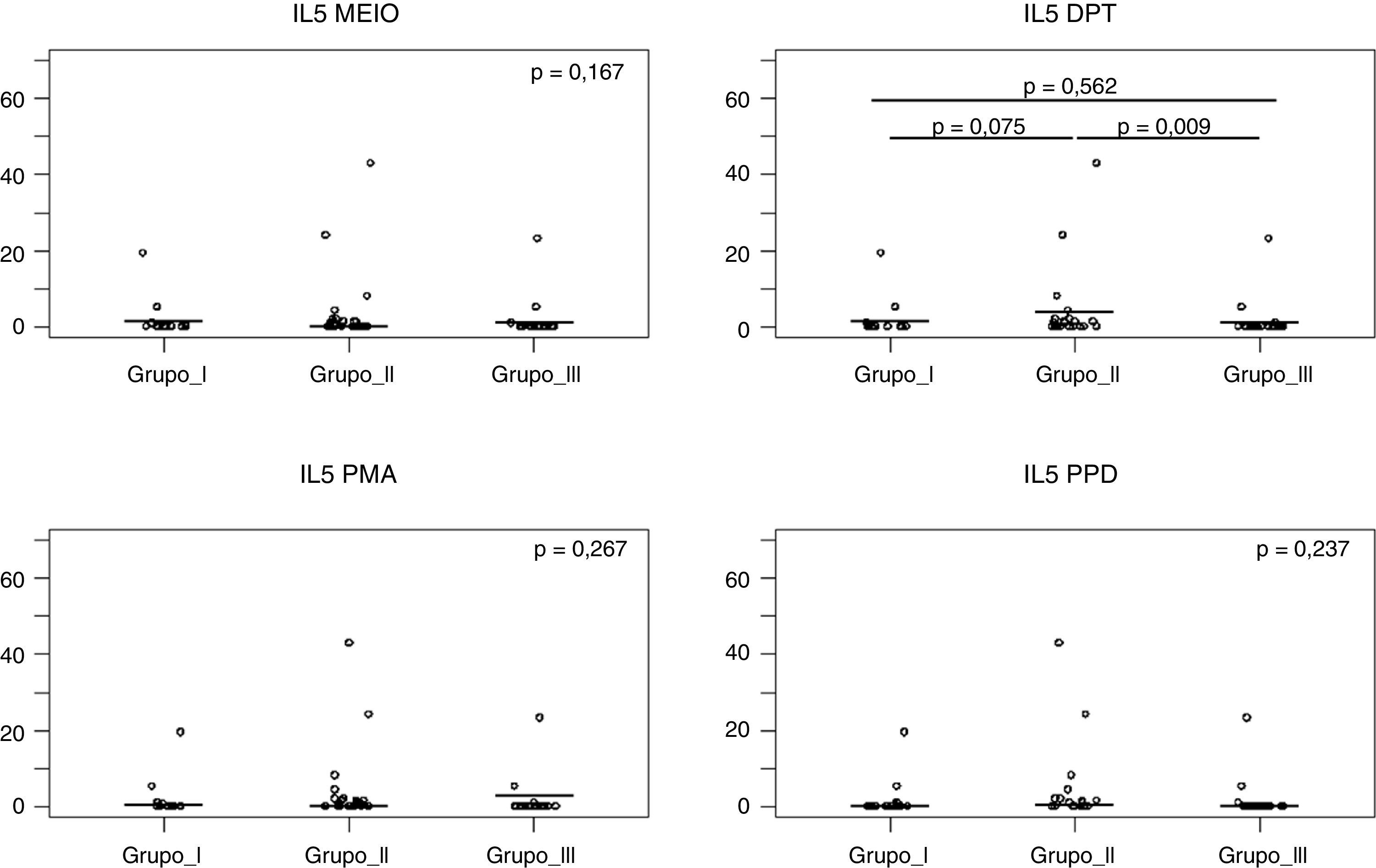
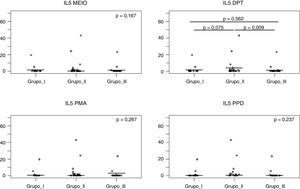
Fig. 2 shows that after Dpt stimulation, the average production of IL-5 levels was higher in the NFWA group than in the NFNA group (p=0.009). In the WFNA group, IL-5 levels were slightly higher only in comparison with the NFNA group (p=0.562), and these levels were lower than those of the NFWA group (p=0.075).
IL-5 levels after 120h of cell culture with stimulation from Dermatophagoides pteronyssinus spt in the three groups studied. Group I (WFNA): with filarial infection and without allergic diseases; Group II (NFWA): without filarial infection and with allergic diseases; Group III (NFNA) – without filarial infection and without allergic diseases.
In the present study, we observed that the immediate hypersensitivity response was similar among subjects with and without allergic disease, including the non-allergic individuals both with and without microfilarial infection. On the other hand, production of IL-5 cytokines when stimulated by D. pteronyssinus was higher (p<0.05) only in the allergic group. The groups had similar profiles of Th2-type response and this seemed to be due both to the current low burden of filarial infection and to the likelihood that these individuals living in an endemic area had previously had parasitic infections. This would have enabled cross-reactivity of parasite antigens and allergens, characterised by high levels of IgE and eosinophils.
The “hygiene hypothesis” suggests that the decline in parasitic infections has led to an increase in allergic diseases.3–6 It can be inferred that, if all allergenic proteins are at equivalent levels in helminth parasites, some may act by inducing production of cross-reactivity antibodies. This is the case regarding some filarial antigens, such as tropomyosins, which are molecularly homologous in invertebrate tropomyosins.28
From a clinical point of view, eosinophilia is an unspecific finding and, as well as in helminth infections, it can be seen in a large variety of non-infectious diseases, such as allergies (including asthma and hypersensitive reactions to drugs), neoplasia, connective tissue diseases and primary hypereosinophilic diseases. However, in areas with prevalent parasitic infections, eosinophilia may be the primary or only indicator of important diseases, such as schistosomiasis or strongyloidiasis.29
The incidence and extent of eosinophilia associated with infection do not depend only on the type, intensity or stage of infection. Other factors, such as individual differences in innate or adaptive immune response, age at first exposure and underlying diseases, are also important. In a previous study on school children in the same area considered here, the prevalence of helminth infection was found to be 64.2%, and approximately 10% presented concomitant filarial infection and intestinal parasitic infections.30 Thus, we can assume that the individuals were probably frequently exposed to intestinal parasitic infection, based on their low socioeconomic status and unfavourable environmental conditions. Moreover, although the presence of intestinal parasites was taken to be an exclusion criterion in this study, the negative predictive value of the stool test was not 100%.31 This may explain the similar frequency of eosinophilia among the three groups studied here.
In all three groups, the frequency of eosinophilia was elevated and was greater than 40% (p=0.52). The frequency of eosinophilia among low-income populations in developing countries is largely linked to chronic and recurring (and often intestinal) helminth infections. Eosinophilia in both helminth infections and allergic diseases (including asthma) is typically associated with a pronounced Th2 immune response. This response includes IL-4, and IL-13 production, as well as IgE production and mobilisation of other specific effector cells, such as mast cells and basophils.29 However, we found that the cellular production of IL-5 after stimulus with D. pteronyssinus was intense in allergic patients.
It is difficult to establish a definition of “normal” regarding total IgE levels in poor regions of the world; in these populations, these levels are frequently elevated due to the population's exposure to a variety of antigens (particularly parasites). As a result, individuals’ immune systems experience from repeated stimulation can affect IgE levels. There was also considerable overlap in total IgE values between participants with and without allergic disease. Therefore, this test cannot discriminate between these individuals.32
Total serum IgE levels were elevated in 57.6% of the participants, with no statistically significant differences among the three groups (p=0.111). In developed countries, asthma is associated with elevated eosinophil levels and elevated total IgE levels. There is also a correlation between the clinical severity of asthma cases and the degree of eosinophilia.33–35 In this study, however, elevated total IgE levels were observed in the NFNA group, which did not present any intestinal parasitic infections or asthma or any other allergic diseases.
In low-income regions in Indonesia, it was found that most seemingly “healthy” children presented markedly elevated IgE levels and eosinophil counts.36 The age group studied here is susceptible to the influence of parasitic infections and infestations, and this factor may explain the significantly altered IgE levels among these individuals.
Although many studies have reported an inverse correlation between helminth infections and skin prick test reactivity to environmental allergens, we only found a few other studies in the literature relating to this possibility and using filarial infection by W. bancrofti, particularly with regard to paediatric populations.37
There have been reports of a strong inverse correlation between filarial infection and responses to skin prick tests: in one study on the lymphatic filarial worm Brugia malayi among Indonesians5 and in another study on the non-lymphatic worm Mansonella perstans among women in Uganda.4 In the present study, no difference in the numbers of individuals with positive results from the hypersensitivity tests was observed between those with and without filarial infection (p=0.49), nor was immunomodulation found for the parameters evaluated in the population with low parasite load (average=22.7mf/ml). The low prevalence of filarial infection in the area of the present study perhaps explains why no individuals with “filarial infection plus allergy” were identified. It was initially envisaged that a group with these characteristics would form part of this study. However, because this is an exploratory study, non-inclusion of this group does not invalidate this results.
The immediate hypersensitivity to airborne allergens was predominantly due to dust mites, as described in the literature.18 In Brazil, epidemiological studies have reported that two dust mite species are prevalent: Dermatophagoides pteronyssinus and Blomia tropicalis.38 Their prevalence is a function of climatic conditions, humidity, temperature and the nutritional makeup of the environment.
In this study, the immediate hypersensitivity response was similar between the filarial infection group (WFNA) and the other two groups studied (p=0.49). The findings regarding infection in the current study differed from those described in relation to infection by S. mansoni.3 The hypothesis of immunomodulation of the allergic response could not be confirmed, because the WFNA group did not present evidence of suppression of the immediate hypersensitivity response.
Eosinophilia was observed in the WFNA group, as were high total IgE levels and an elevated response to hypersensitivity tests. IL-4 production in this group was found to be similar to that of the allergic disease group, though there were no clinical manifestations. The elevated responses to the IgE test and the hypersensitivity test in the group with chronic filarial infection may be explained by the cross-reactivity of filarial antigens (such as tropomyosin from W. bancrofti) with the homologous protein from dust mites (Dpt 10).6 In addition, because of the regulatory environment (particularly in the case of chronic infection with IL-10 and TGF-β production), allergies and their clinical manifestations are regulated and suppressed in these individuals, despite the elevated specific IgE allergen antibody levels found.
When stimulated by Dpt, IL-5 production was found to be higher in the NFWA group than in the NFNA group or in the WFNA group. This may be a more important and specific profile regarding the allergic response to this house dust mite.
The results regarding the response to immediate hypersensitivity in the present study are closest to the real situation in which filariasis, geohelminths and allergic diseases coexist. Thus, the immune response observed is a “sum”, which translates memories of previous repeated infections.
In summary, this study did not show any association between skin response to allergens and lymphatic filarial infection by Wuchereria bancrofti in individuals with low worm burden. It showed that the groups studied had similar profiles for Th2-type response, except regarding IL-5 cellular production after stimulus with D. pteronyssinus, which was exacerbated in allergic patients.
Conflict of interestThe authors have no conflict of interest to declare.
This work was supported by the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico), under procedural no. 476336/2008-2. We would like to thank Dr. Maria Ilma Araújo for the donation of D. pteronyssinus extract, and Mrs. Roberta Brito for the skin prick tests performing and Dr. Armenio Aguiar for suggestions and reviewing of the text.