House dust mites (HDM) are arthropods of medical importance due to their relationship with allergic diseases. House dust provides a detrital habitat for these organisms, in which human skin scales are a primary food source. For digestion, wall gut cells elaborate potent proteases.
Nevertheless, the observation of flagellated protozoa in intestinal extracts of HDM by light microscopy might contribute to digestive processes in mites, opening a new avenue of research regarding the ecological interactions between mites and these microorganisms in the utilisation of such substrates, as well as with regard to allergic diseases.
House dust mites (HDM) are very small arthropods that are barely visible to the unaided eye (0.2–0.5mm in length). They belong to the genus Dermatophagoides, family Pyroglyphidae, and order Astigmata, and are closely associated with allergic diseases. Medically-important HDM species principally include Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Euroglyphus maynei.1 These mites are widespread in nature, living principally in warm and specific humid environments such as on mattresses, bedding, upholstered furniture, carpets, and curtains.2 Moreover, dust mites can be transferred from home to home via human skin and clothing. House dust provides a detrital habitat for the mites, containing three key macromolecules that are derived from organic debris: keratin (human and pet skin scales, hair, and nails), cellulose (textile fibres), and chitin (fungal hyphae and mite cuticles).3 Nevertheless, the HDM diet is also composed of organic fibres (e.g., wood and cotton), bacteria, pollen grains, fungal mycelia, and the spores of microorganisms.4
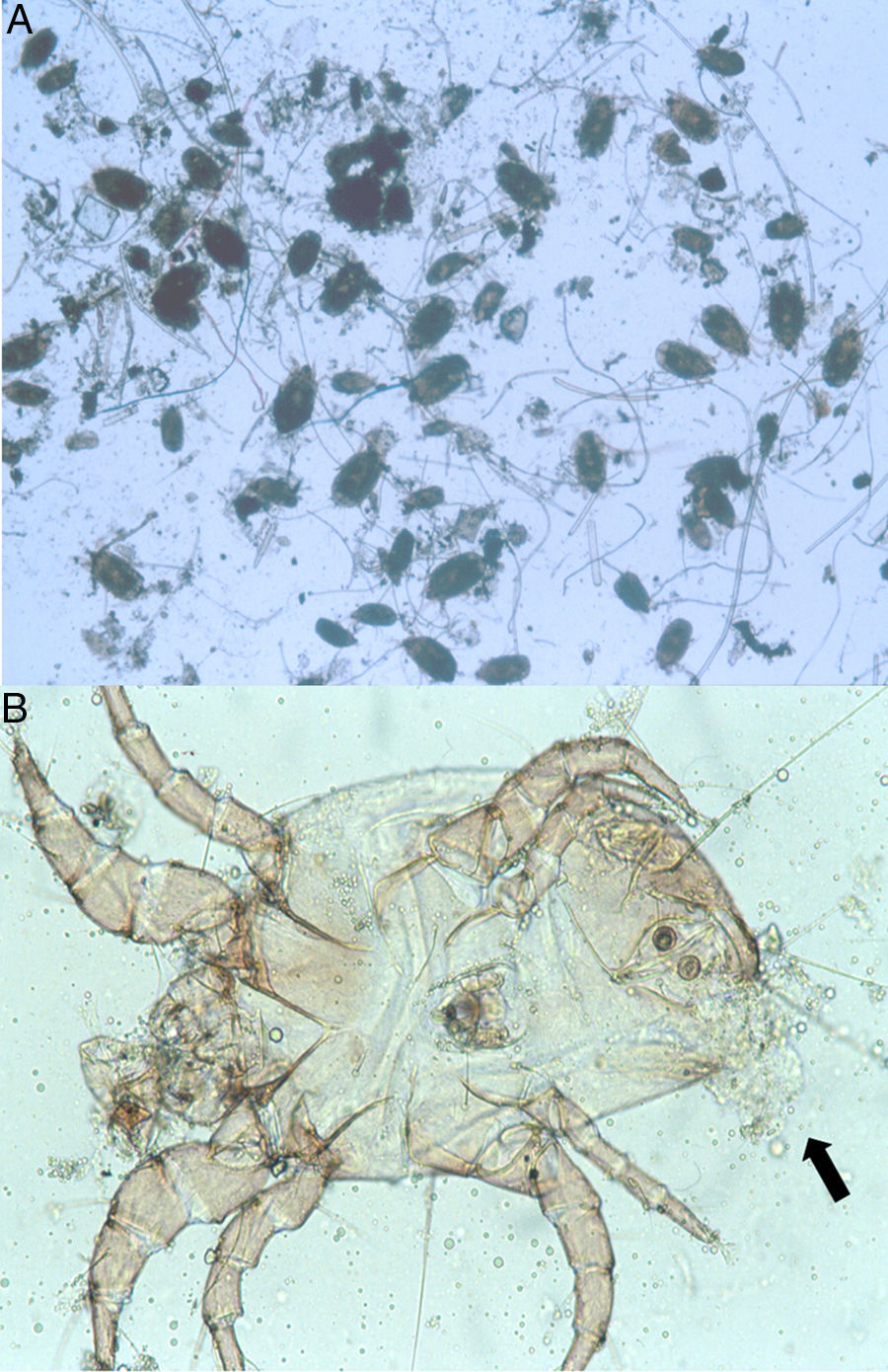
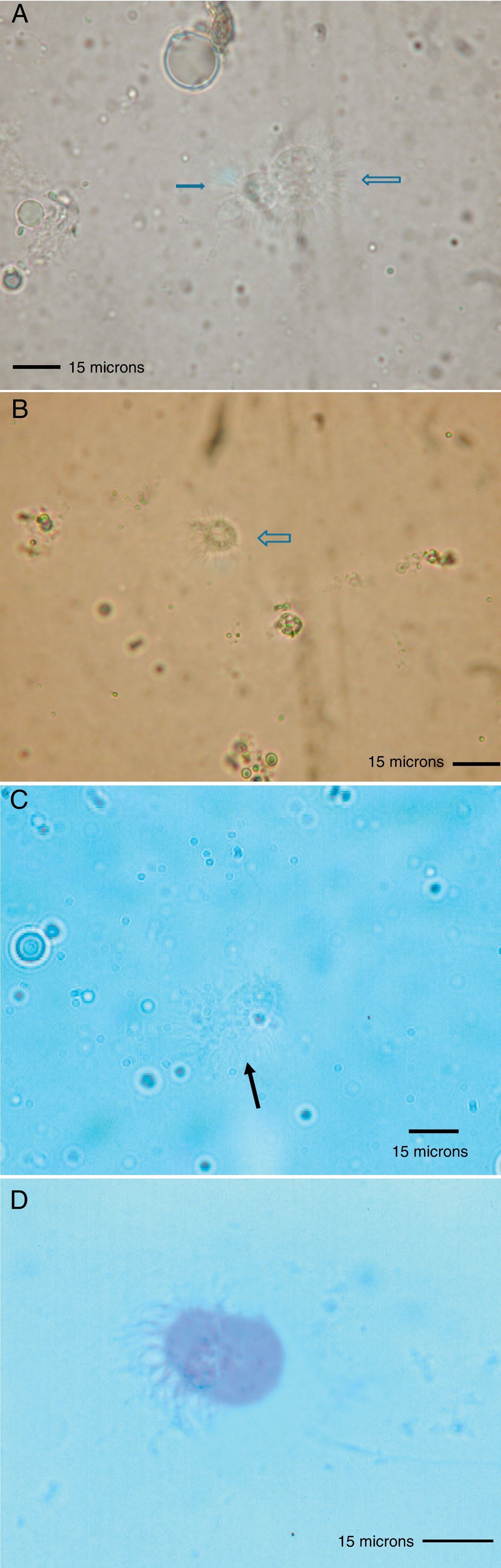
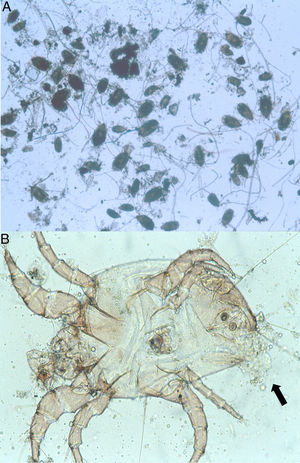
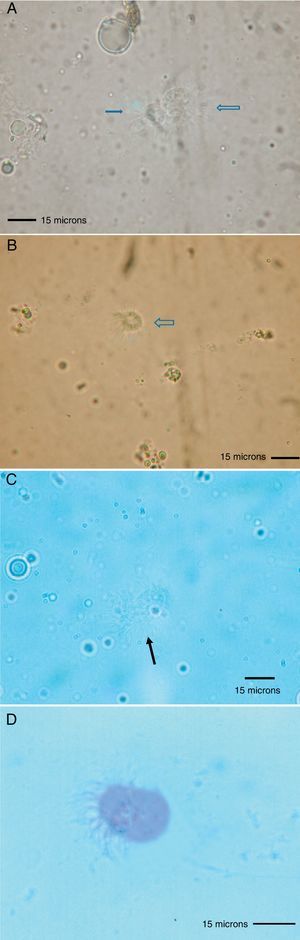
Because the small size of HDM complicates digestive studies and dissection of their gut is very difficult in comparison to other types of arthropods,5 squash preparations of the mites to observe intestinal extracts under a light microscope constitute a simple, cost-effective, and reliable procedure for examining the intestinal contents of HDM. A variety of components can be observed under a compound microscope, such as macerated skin scales, fungal hyphae and yeast, spores, bacteria, pollen grains, cotton fibres, and many unidentifiable particles, although the presence of flagellated protozoa has not been mentioned.4 Nevertheless, the presence of flagellated protozoa in intestinal extracts from house dust mites has been previously described.6 Therefore, to compare this finding to that reported by Colloff,4 the experiment was repeated with slight differences (utilisation of a centrifuge and a protozoal stain such as Wheatley's trichrome). Vacuum cleaner dust was sprinkled onto standard 25mm×75mm glass microscope slides (Fig. 1A). Under a light microscope and with the aid of thin histological needles, HDM were collected (only adult forms) and added to a centrifuge tube containing distilled water (ten mites per tube). Centrifugation was performed at 1200rpm/5min. The supernatant was decanted to obtain specimens without pollutants. Thus, each mite was placed in a drop of distilled water on a glass microscope slide and squashed with a cover slide to observe the intestinal contents under a light microscope (Fig. 1B). The intestinal extracts were observed under oil immersion to search for the presence of flagellated protozoa. The observed forms were round to ovoid in shape, ranging in size from 8 to 12μm, with filiform projections in the form of flagella (Fig. 2A–D).
Several years ago, different types of bacteria (Bacillus sp., Staphylococcus sp., Gram-negative non-fermenting rods and coryneform Gram-positive rods) and different species of fungi (Aspergillus sp., Penicillium sp., Cladosporium sp., Alternaria sp., Acremonium sp., Paecilomyces sp., and yeasts) were isolated from mite homogenates of D. pteronyssinus and D. farinae.7 Based on bacterial 16S rRNA gene sequences and fluorescence in situ hybridisation, new species-specific bacteria (Rhizobiales, Bacteroidales, Enterobacteriales, Bacillales and Actinomycetales) were identified and located in the digestive tract of synanthropic mites (A. siro, D. farinae, L. destructor, and T. putrescentiae).8 Moreover, DNA and RNA sequencing samples revealed a diverse endosymbiotic microbiome (Enterobacter sp., Staphylococcus sp., Escherichia sp., among others) in the mite D. farinae, confirming the abundance of enterobacteria in the intestine of this mite.9
In contrast, several species of fungi (e.g., Eurotium amstelodami, Aspergillus penicillioides, and Wallemia sebi) are considered to be inhabitants of the gut of D. pteronyssinus,10 another important factor in the house dust ecosystem.11
Considering these findings, interactions between the gut of laboratory cultured domestic mites and bacterial or fungal microorganisms have been proposed by some authors as essential for effective utilisation of nutrients in the house dust environment.12
However, the success of the flagellated protozoa in intestinal extracts of HDM has yet to be explained, raising two questions. First, are these protozoa symbiotic or harmful to the HDM? Second, do these flagellated protozoa play a role in respiratory allergy?
Regarding the first question, among other types of wood roach arthropods such as the so-called lower termites, the major players in the digestion of biomolecules such as cellulose are unique lineages of cellulolytic flagellates. Nevertheless, a dual decomposition system consisting of the termite's own cellulases and those of its gut protists have been elucidated at the molecular level.12 Thus, these flagellated protozoa might similarly contribute to the digestive processes in the intestine of HDM.
Concerning the second question, Toll-like receptors on lung epithelia may be triggered by certain components of microbes and contribute to the development of allergic reactions to different household antigens.13 The flagellated protozoa observed in the intestinal extracts of HDM could act as triggers for these microbial receptors on the airway epithelium. In support of this hypothesis, some studies have highlighted this phenomenon in terms of the damage to the respiratory epithelium.14,15
Thus, further studies (cultures and molecular techniques) are necessary to elucidate important features of these flagellated protozoa in intestinal extracts of HDM, such as their taxonomic, biological, and ecological significance, their presence in all HDM species, and their potential role(s) in the pathogenesis of allergic diseases, among others.
Ethical disclosuresConfidentiality of dataThe author declares that no patient data appears in this article.
Right to privacy and informed consentThe author declares that no patient data appears in this article.
Protection of human subjects and animals in researchThe author declares that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThere is no conflict of interest in submission of this manuscript.