The present study was designed to explore the possible changes in endogenous hydrogen sulphide (H2S), a novel gasotransmitter, on the pathogenesis of allergic rhinitis (AR).
MethodsAR guinea pig model was established by nasal ovalbumin sensitisation. Guinea pigs were divided into four groups: Saline control, AR sensitised, sodium hydrosulphide (NaHS) treated, and propargylglycine (PPG) treated group. The frequency of sneezing and nose rubbing was recorded. Leukocyte infiltration in nasal lavage fluid (NLF) and plasma H2S level were measured. Expression of Cystathionine-β-synthase (CBS) and Cystathionine-γ-lyase (CSE) mRNA as H2S-producing enzymes in nasal mucosa was determined by real time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR).
ResultsThe frequency of sneezing and nose rubbing, and levels of leukocyte infiltration in NLF were higher than those of control (P<0.01), but plasma H2S in sensitised guinea pigs was lower than those of control (P<0.05). From the results of RT-PCR, it was found that the expression of CSE was higher than CBS in nasal mucosa, and in sensitised guinea pigs it was lower than that of control (P<0.05). NaHS successfully increased the level of H2S and alleviated the symptoms of AR accompanied by up-regulation of CSE as compared with AR group (P<0.05). PPG significantly suppressed the expression of CSE and decreased the H2S level, yet also aggravated the symptoms of AR.
ConclusionH2S level may be negatively correlated with the process of inflammation and positively correlated with expression of CSE in nasal mucosa. The endogenous H2S pathway is down-regulated in AR.
Rhinitis, especially allergic rhinitis (AR), continues to be a major health problem and although some treatments are available, none is ideal. Research on the role of the gas signal messenger such as nitric oxide (NO) and carbon monoxide (CO) in allergy medicine is a rapidly emerging field.1,2 However, the molecular mechanisms of AR are still poorly understood, and researchers are seeking novel endogenously produced gasotransmitters to investigate their possible roles in the pathogenesis of allergic inflammation. Recently, hydrogen sulphide (H2S) was found to be the third endogenous signalling gasotransmitter because of its endogenous metabolism and physiologic functions.3 Now H2S is increasingly recognised as a member of a growing family of “gasotransmitters”, together with its two counterparts, NO and CO.
H2S was only recognised as a kind of toxic gas in contaminated environments with a strong odour of rotten eggs for a long time, and its major effects were intoxication of the central nervous system and inhibition of respiratory system. Endogenous H2S may be generated by two pyridoxal-5′-phosphate-dependent enzymes: cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) in mammalian tissues, which use L-cysteine as the main substrate. The expressions of these two enzymes are tissue-type specific.4,5 H2S is directly produced in myocardial tissues, arterial and venous tissues by CSE,6–8 and in some tissues such as the nervous system, CBS is only needed for the generation of H2S.9 Otherwise, the expressions of CBS and CSE are both identified in several mammalian tissues, including liver, kidney, brain, ileum, and blood lymphocytes.3,10
Recent data suggest that H2S may contribute to many inflammatory processes such as asthma,11 acute pancreatitis,12 endotoxaemia,13 and COPD.14 This demonstrated that H2S plays a key role in modulating leukocyte adhesion to vascular endothelium, leukocyte infiltration, and oedema formation, which are characters of inflammation.
To clarify the role of endogenous H2S in the pathogenesis of AR, we investigated the level of plasma H2S in guinea pigs with AR. Clinical symptoms of animals such as sneezing and nose rubbing, and leukocyte infiltration in nasal lavage fluid (NLF) were studied as non-invasive markers of inflammation. The expressions of CBS and CSE of nasal mucosa were also studied by real time RT-PCR. Sodium hydrosulphide (NaHS) and propargylglycine (PPG) were used as donor and inhibitor of H2S respectively in our study,15 and PPG is a specific inhibitor of CSE, which can suppress H2S production in tissues.16 Through regulated H2S level by NaHS and PPG, we investigated the changes of H2S on the inflammation process of AR.
Materials and methodsMaterial and animal modelTwenty-four aged healthy male Hartley guinea pigs, weight 230–280g (National rodent laboratory animal resources, Shanghai branch, China) were taken and divided into four groups: Saline control group, AR sensitised group, NaHS treated group, and PPG treated group. The animal models of allergic rhinitis were prepared according to the methods made by Al Suleimani M et al.17 Guinea pigs (n=18) were initially exposed to 1% ovalbumin (10mg/kg, Sigma Inc.MD ) in saline given as a 1% aerosol twice for 10min each, 7 days apart. On days 14, 15 and 16, a booster of 1% ovalbumin in saline was instilled intranasally at a volume of 20μl/nostril/day into both nostrils. On day 21 guinea pigs were challenged with 2% ovalbumin in saline instilled intranasally at a volume of 20μl/nostril in each nostril. Eighteen sensitised guinea pigs were divided into three groups, one was continually treated with OVA as AR group. In the second group, named as NaHS group, animals (n=6) were intraperitoneally administered NaHS (Sigma Inc. MD) at a dose of 14μmol/kg/day, 12h after every nose inspiration with ovalbumin and continually for two weeks. In the third group, named as PPG group, animals (n=6) were intraperitoneally administered PPG (Sigma Inc.MD) at a dose of 30mg/kg/day immediately after every nose inspiration with ovalbumin continually for two weeks too. Control animals (n=6) were challenged in a similar manner by using saline.
Observation of sneezing and nose rubbing and assessment of leukocyte infiltrationFrequency of sneezing and nose rubbing were assessed as previously described by Al Suleimani M et al. with modifications.17 They were counted directly following nasal challenge, and for 30min thereafter. A sneeze was characterized by an explosive expiration just after deep inspiration and an external perinasal scratch with the animal's forelimbs characterized a nose rub. NLF was collected from guinea pigs 1h post-challenge as follows17: nasal cavities were washed with 2ml of pre-warmed saline infused from the tracheal side. NLF was collected from the anterior naris and total cell count was assessed using a standard haemocytometer. Leukocytes were counted under light microscope at power 40×, and the following formula was used:
Number of cells/ml=total number of cells counted×dilution factor×1000/total volume counted (0.1mm3).
Measurement of plasma H2S concentrationGuinea pigs were anaesthetised by intraperitoneal administration of pentobarbital (40mg/Kg). 1ml blood was collected from heart through direct cardiac puncturation, avoiding air contact. A sample of plasma (0.1ml) was added to a test tube containing 0.5ml of 1% zinc acetate and 2.5ml of distilled water, then 0.5ml of 20mmol/L N,N-dimethyl-p-phenylenediamine dihydrochloride in 7.2mol/L HCl and 0.4ml of 30mmol/L FeCl3 in 1.2mol/L HCl were also added to the same test tube for 20min of incubation at room temperature. The protein in the plasma was removed by adding 1ml of 10% trichloroacetic acid to the solution and centrifuging it. The optical absorbance of the resulting solution at 670nm was measured with a spectrometer (Lambda Bio, Perkin Elmer Inc, MD). H2S concentration in the solution was calculated against the calibration curve of the standard NaHS solution.
Total RNA extraction and cDNA synthesisThe guinea pigs were sacrificed by rapid decapitation. Biopsies of nasal mucosa were taken from the inferior turbinate and put in liquid nitrogen immediately. They were then minced with a scalpel on dry ice and transferred immediately to 2ml polypropylene tubes, homogenised and total RNA was extracted using Trizol™ reagent (Invitrogen Inc, MD) following the manufacturer's instructions. The concentration and purity of RNA were determined spectrophotometrically. Then the synthesis of cDNA was performed according to a cDNA synthesis kit (PrimeScript RTase, TaKaRa Inc, Japan).
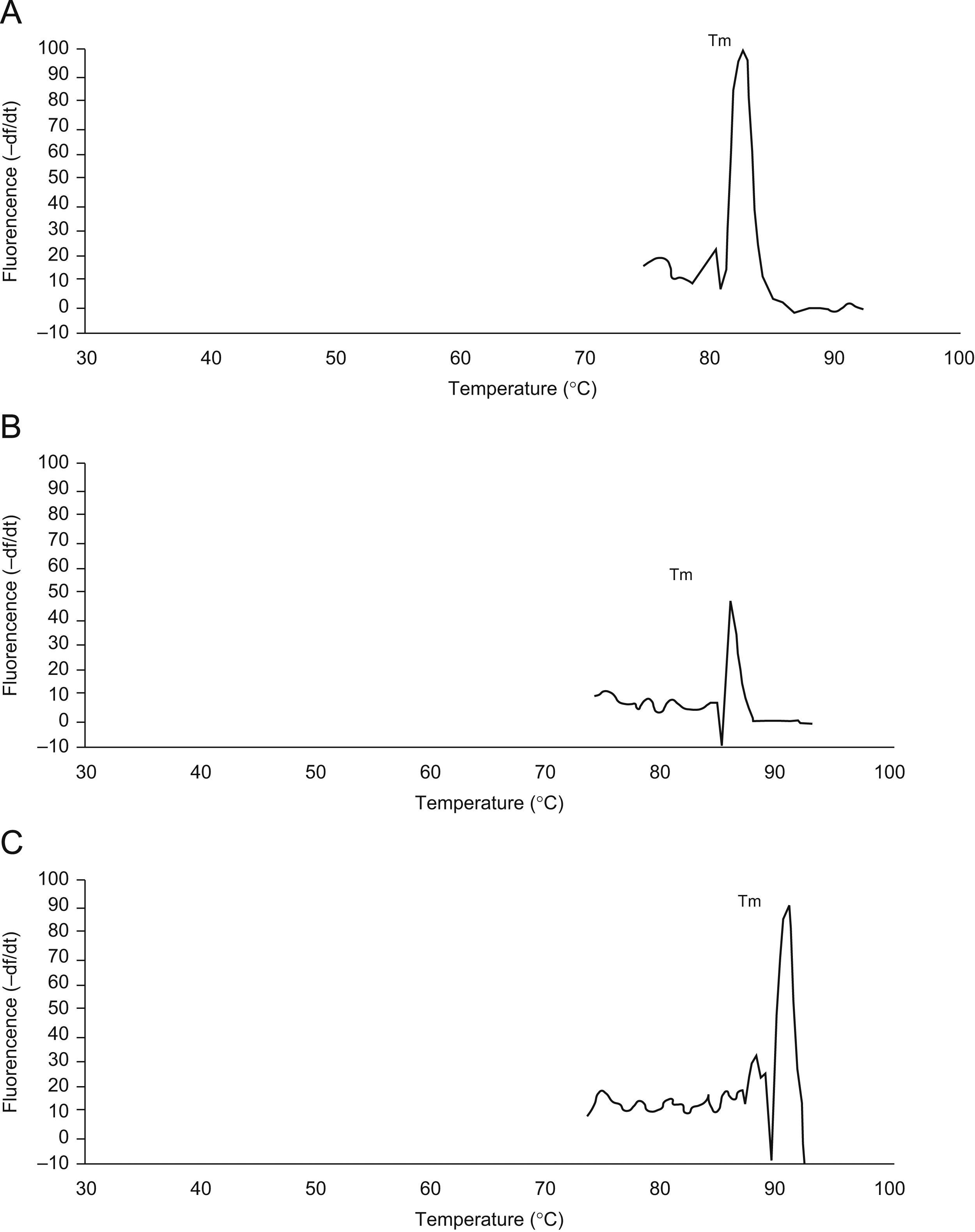
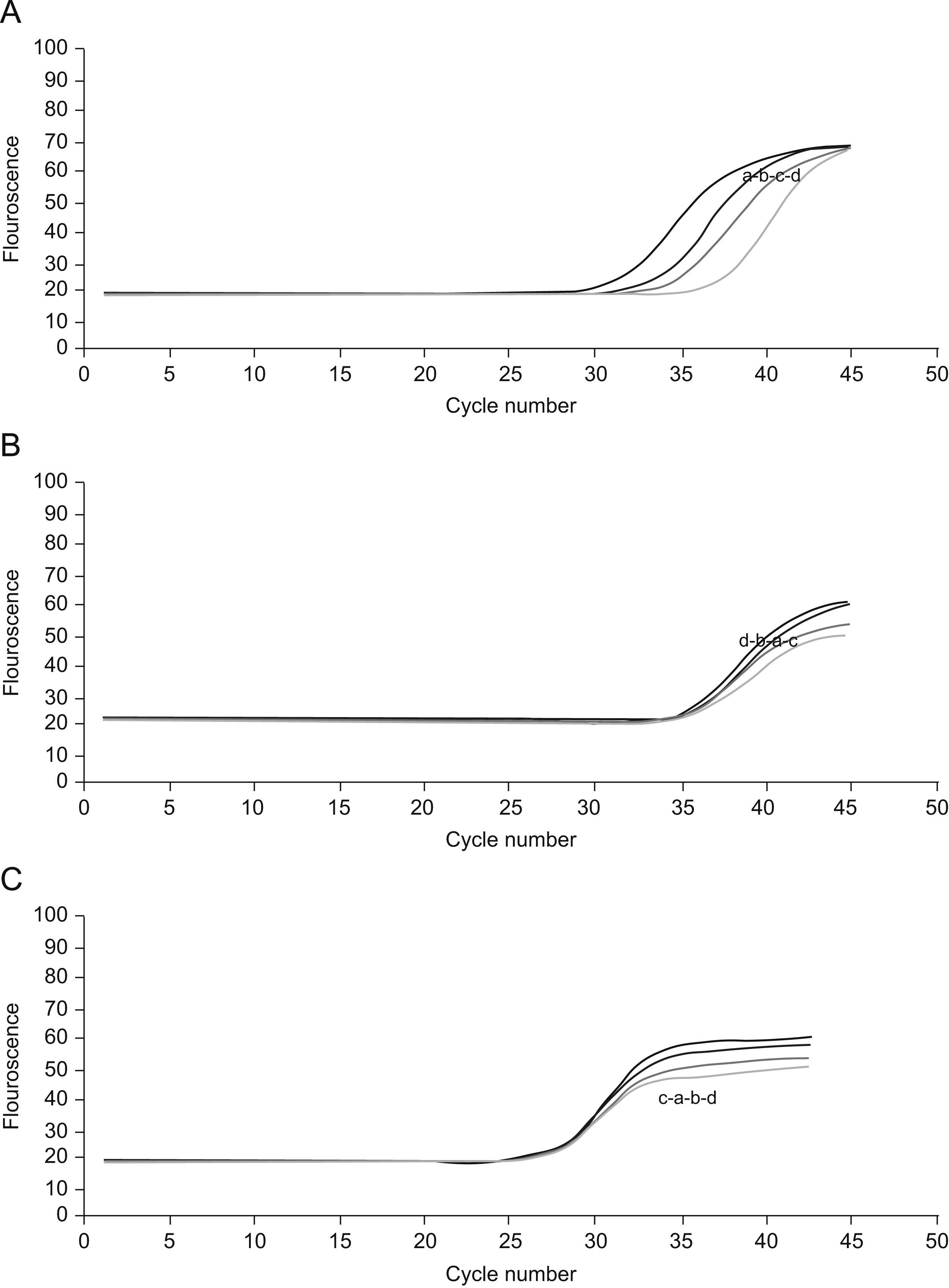
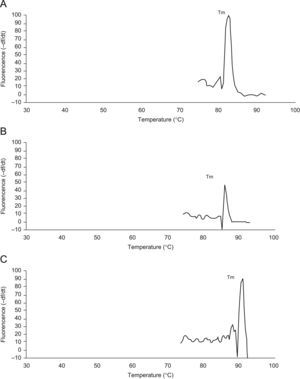
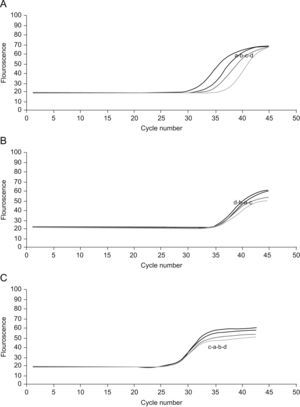
Real time reverse transcriptase-polymerase chain reaction for CBS and CSE mRNA expressionsTo determine the expressions of the CBS and CSE gene in nasal mucosa, fluorescent quantitative real time RT-PCR assay was performed. The sequences of the primers (TaKaRa Inc, Japan) specific for CBS and CSE were performed with sense (CBS: CCAGGACTTGGAGGTACAGC, CSE: TCCGGATGGAGAAACACTTC) and antisense (CBS: TCGGCACTGTGTGGTAATGT,CSE:GCTGCCTTTAAAGCTTGACC) primers, with an expected size of the amplified sequence of 155bp for CBS and 400bp for CSE. β-actin was used as control (sense: ACCCTTAAGGCCAACCGTGAAAAG, antisense: TCATGAGGTAGTCTGTC AGGT, 240bp). Then the incubation of cDNA and primer was performed at 95°C for 5min and the PCR reaction proceeded for 45 cycles: 95°C for 20s, 57°C for 20s, and 72°C for 20s in a programmable thermal cycler (Line-Gene real-time PCR detection system, bioer Inc, China) using a thermostable Taq DNA polymerase (SYBR PrimeScript Ex Taq, TaKaRa Inc, Japan) final incubation at 72°C for 7min. Fluorescent product was measured by a single acquisition mode at 86°C after each cycle. After the completion of PCR amplification, a melting curve analysis was performed. Fig. 1 shows a sharp peak with a melting temperature (Tm) of CBS(Tm A) of 86°C, CSE(Tm B) of 84°C and β-actin (Tm C) of 90°C. For each sample, the amount of both target and endogenous control (β-actin, a housekeeping gene) were determined. The typical amplification curves of real-time RT-PCR for CBS, CSE and β-actin mRNA are shown in Fig. 2. The amount of the target molecule was then divided by the amount of the endogenous reference, to obtain a normalised target value. The PCR products were also run on 1.5% agarose gel and visualised by ultraviolet light.
Cycles of CSE(A), CBS(B) and β-actin(C).For the four curves: control group(a), NaHS group(b), AR group(c) and PPG group(d).The vertical axis represents the degree of amplification by SYBR-Green fluorescence and the horizontal axis represents the number of amplification cycles. With the same cycle number, the groups have similar amplification of β-actin and CBS but different amplification of CSE.
All data were expressed as mean±S.D. Statistical analyses of data were performed using ANOVA for multiple comparison and LSD for comparison among groups, and Pearson Correlation for the two-variable correlation analysis. P<0.05 was considered to be statistically significant.
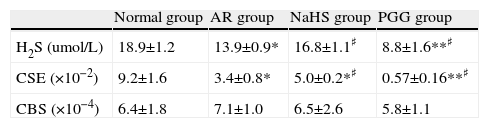
ResultsConcentration of H2S in plasmaThe blood H2S level of the AR group was lower than that of the non-sensitised group (p<0.01). H2S level increased significantly after being treated with H2S donor NaHS, and decreased after PPG was administrated, as compared with AR group (p<0.05) (see Table 1). It indicated that down-regulation of H2S level existed in AR, and NaHS increased H2S level and PPG decreased it successfully.
| Normal group | AR group | NaHS group | PGG group | |
| H2S (umol/L) | 18.9±1.2 | 13.9±0.9* | 16.8±1.1♯ | 8.8±1.6**♯ |
| CSE (×10−2) | 9.2±1.6 | 3.4±0.8* | 5.0±0.2*♯ | 0.57±0.16**♯ |
| CBS (×10−4) | 6.4±1.8 | 7.1±1.0 | 6.5±2.6 | 5.8±1.1 |
The concentration of H2S of Plasma, eotaxin of nasal lavage fluid, expression of mRNA of CSE and CBS of nasal mucosa. All data represent as mean±S.D.*,**: Significantly different from the control group (p<0.05 and p<0.01, respectively). ♯,♯♯ Significantly different from the AR group (p<0.05 and p<0.01, respectively).
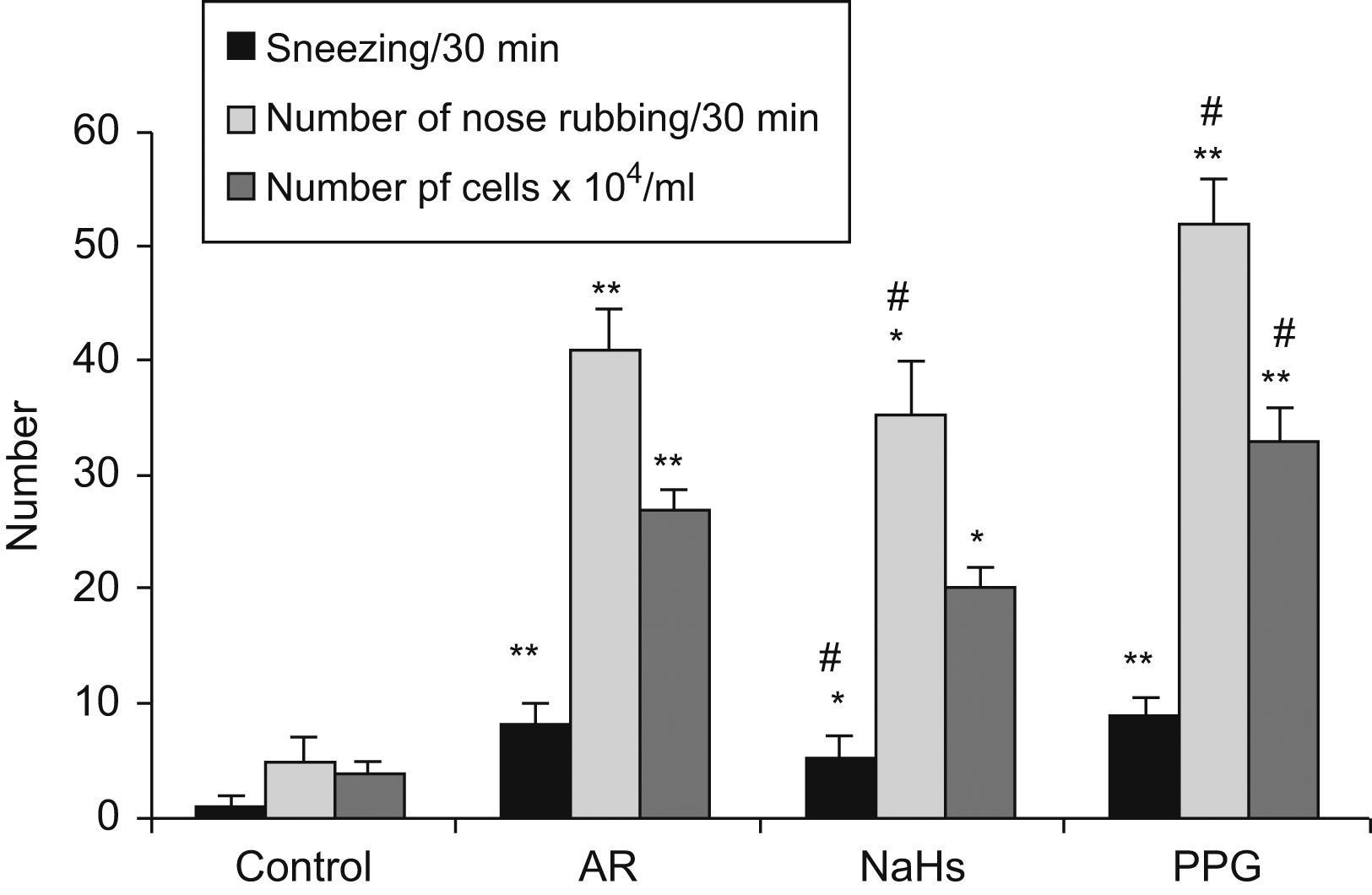
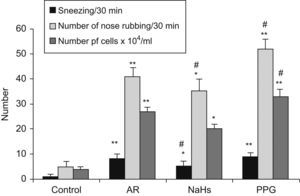
The results are shown in Fig. 3. Sneezing frequency and number of nose rubbings in sensitised AR group were significantly increased (p<0.01) as compared with a non-sensitised group, and increased further in PPG treated group as compared with AR group (p<0.05), but significantly decreased in NaSH treated group (p<0.05). In AR group, there was a significant increase of total cell count in NLF (p<0.01), especially eosinophils and neutrophils as compared with non-sensitised groups. Total cell count significantly increased after PPG treated, and decreased after NaHS treated (p<0.05), as compared with AR group. It indicated that NaHS increased the H2S level but reduced the inflammatory response of allergy e.g. inhibited leukocyte infiltration in nasal mucosa, but PPG had the opposite effect on allergy.
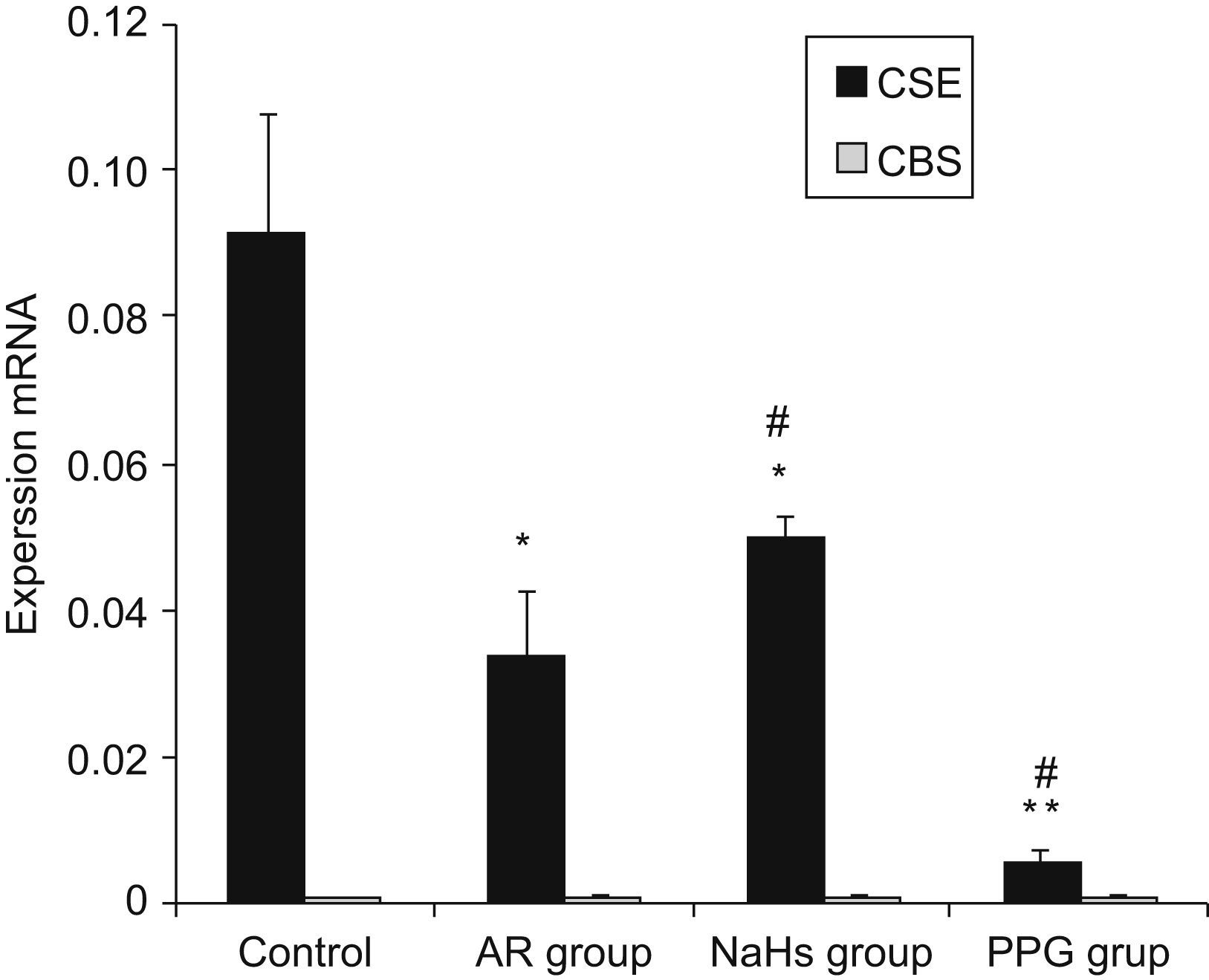
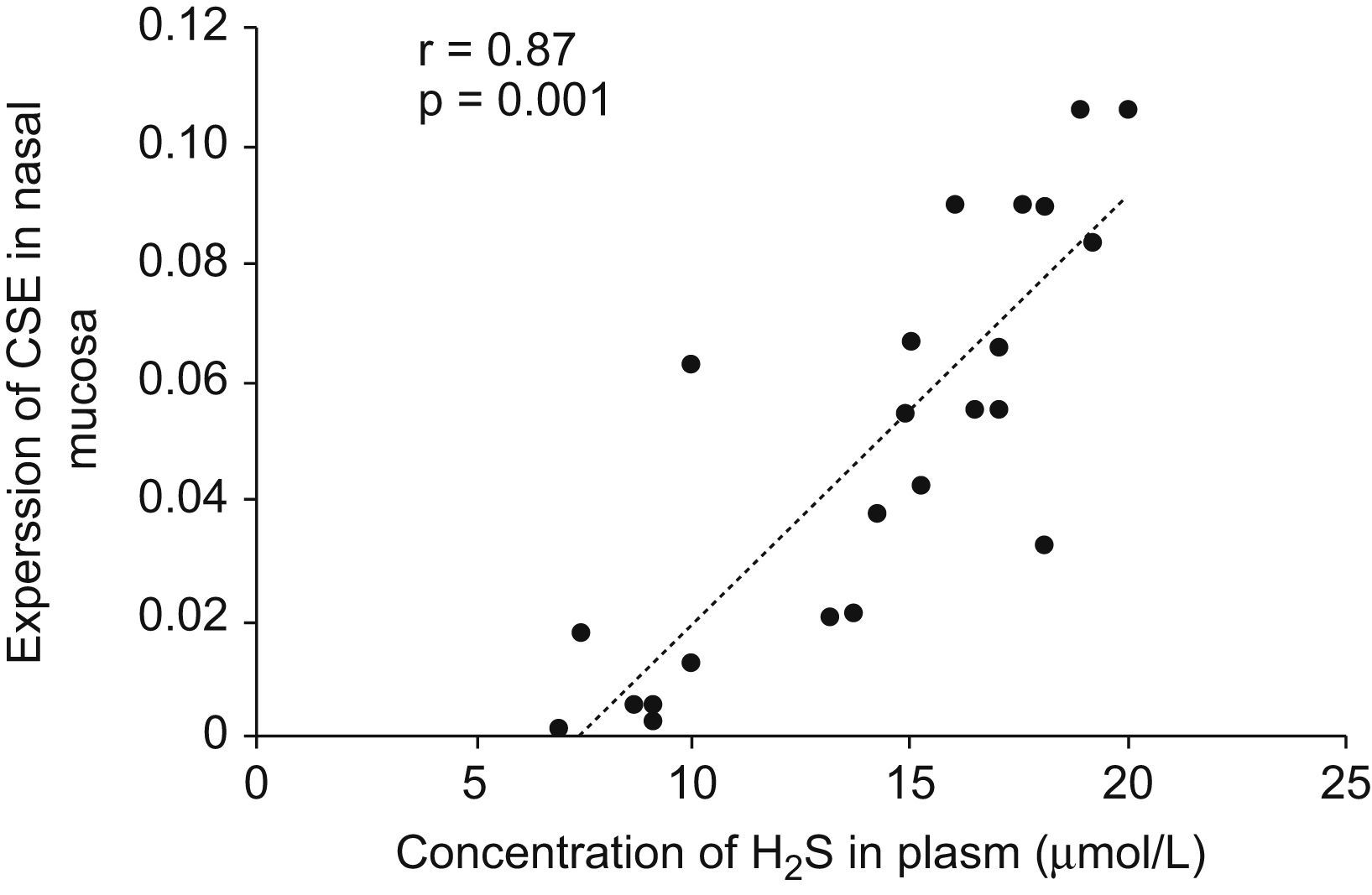
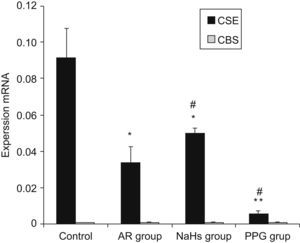
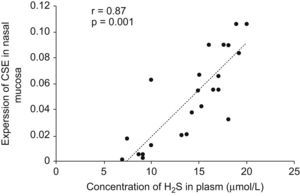
Expressions of CBS and CSE by real-time RT-PCRThe cumulative data for mRNA expressions of CBS and CSE are presented in Table 1 and Fig. 4. CSE mRNA expression was down-regulated significantly in AR group as compared with control (p<0.05) and the expression was increased significantly after being stimulated by NaHS (p<0.05), and decreased significantly with PPG administration as compared with AR group (p<0.05). The expression of CBS in nasal mucosa was very weak and no significant changes of CBS mRNA levels were observed between groups (p>0.05). To verify the amplification curve results, representative samples of the PCR products were run on 1.5% agarose gels. Electrophoresis results showed that the order of CSE mRNA expression levels from high to low was control, NaHS, AR and PPG group (Fig. 5). Moreover, correlation between the level of CSE mRNA and concentration of H2S was also analysed. There was a high significant direct relationship between them (r=0.87, P=0.001) (Fig. 6). It suggested that the level of H2S was positively correlated with CSE of nasal mucosa through a concentration-dependent manner and that the NaHS and PPG regulated the level and might have a relationship with the expression of CSE of nasal mucosa in AR. The CBS of nasal mucosa perhaps had little effect on level of H2S in AR.
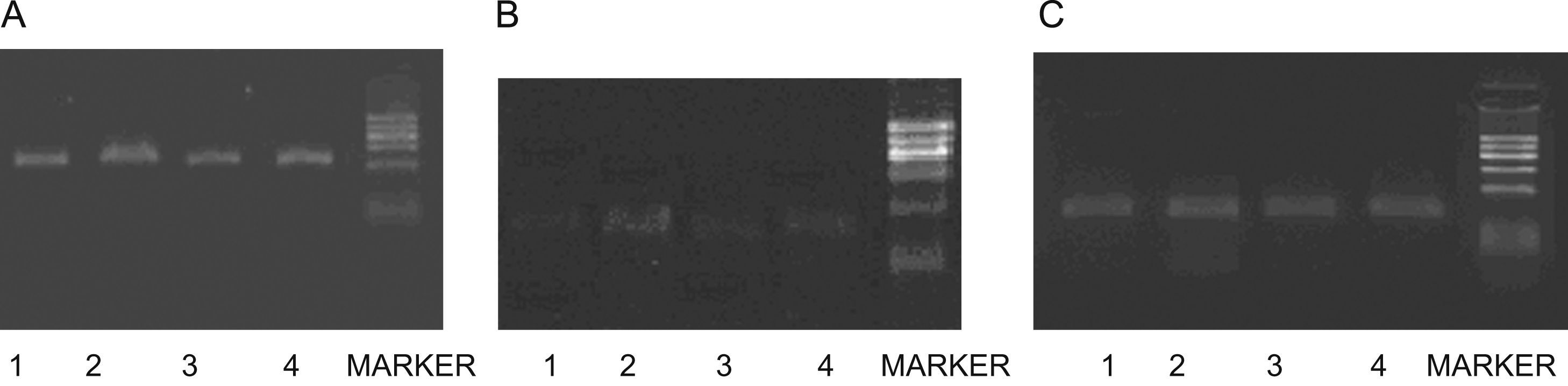
Image of gel of RT-PCR for CSE(A),CBS(B) and β-actin(C) mRNA from nasal mucosa of guinea pigs. Sizes of PCR products were 400bp(CSE),155bp(CBS) and 240bp(β-actin).Lanes 1–5 (from left to right) were products of AR, control, PPG, NaHS groups and DNA marker (100∼600bp).There was a decrease in CSE cDNA in AR group compare with control, and it increased after NaSH treated and decreases after PPG treated, whereas there was no change in β-actin mRNA. The expressions of CBS mRNA were so weak that no significant difference between groups was found.
Correlation between concentration of H2S in plasma and CSE mRNA expression level in nasal mucosa. Pearson Correlation was used to analyse the relationship between the level of H2S and expression of CSE mRNA. There was a highly significant direct relationship between them (r=0.87, P=0.001).
After nasal provocation with ovalbumin, the AR was evaluated from the occurrence of typical clinical symptoms with respect to nose and eyes irritation, like sneezing, conjunctival and nasal secretion. In comparison to saline, ovalbumin sensitisation increased sneezing frequency and numbers of nasal rubs. Nasal cellular infiltration (extravasation of leukocytes) is a characteristic hallmark of AR. Following nasal allergen challenge in sensitised guinea pigs, there was a significant increase in total cell count (p<0.01) as compared with non-sensitised groups. Furthermore, both eosinophils and neutrophils were significantly induced. Sneezing frequency, number of nasal rubs, and total cell counts in nasal washings can be used as indices of allergic response.
This study was the first to show down-regulation of endogenous H2S in sensitised AR guinea pigs. Previous studies had shown that gaseous transmitters, NO and CO, played important roles in the pathogenesis of AR.18,19 H2S is a colourless and flammable gas with a small molecular weight, and now is increasingly recognised as a member of a growing family of “gasotransmitters”, together with its two counterparts, NO and CO. It was only recently that researchers came to understand H2S as a novel gasotransmitter playing an important biological role, especially in airway inflammation such as in asthma, COPD, or lung injury.12,14
H2S is produced mainly by two pyridoxal-5’phosphate-dependent enzymes responsible for the metabolism of L-cysteine, CBS and CSE. It should be noted that H2S generation is closely associated with the catabolism of cysteine and methionine as well as with glutathione metabolism. Both CBS and CSE are responsible for the metabolism of methionine into cysteine, which is in turn used for the generation of H2S,20 but the two enzymes differ in the specific mechanism of H2S formation. CSE catalyses the conversion of cystine (a cysteine disulphide) to thiocysteine, pyruvate and ammonia, thiocysteine then non-enzymatically decomposes to cysteine and H2S. The major mechanism of H2S produced by CBS probably involves the condensation of homocysteine with cysteine to yield cystathionine, and H2S is released during this reaction.21,22 The expressions of CBS and CSE had been identified in many human and other mammalian cells, including those from liver, kidney, brain, skin fibroblasts, and blood lymphocytes. In some tissues CBS and CSE are both needed for generation of H2S, whereas in others only one enzyme is needed.4–8 Thus, it has come to be known that the expression of CBS and/or CSE is tissue specific. In nasal mucosa, more expression of CSE was found but not of CBS, and the mRNA expression of CSE was positively correlated with the concentration of H2S by concentration-dependent manner, so the CSE was maybe the major H2S-producing enzyme in nasal mucosa and it was positively correlated with the level of H2S in AR, because CSE mainly exists in vascular smooth muscle cells (SMC),23 and expressed in nasal mucosa might be attributed to the rich distribution of vascular SMC.
In order to investigate the influence of H2S level on symptoms of AR, NaHS was used as H2S donor. NaHS can be dissociated to Na+ and HS− in solution, and then HS− associates with H+ and produces H2S.24 In the experiment after NaHS treatment, H2S level was successfully up-regulated in plasma as compared with the AR group (p<0.05), and it alleviated the symptoms of AR. PPG was used as an inhibitor of H2S. Our observations had shown that PPG significantly attenuated the expression of CSE, and in the PGG group the level of H2S was decreased significantly as compared with the AR group. PPG can suppress the production of H2S by inhibited CSE, and symptoms of AR were aggravated after PPG treatment, accompanied by enhanced leukocyte adhesion, leukocyte infiltration, perhaps with oedema formation. All these suggested that the level of H2S has a negative effect on symptoms of AR.
In mammalian tissues H2S may be a physiological regulator with its vascular effect possibly mediated by the opening of the KATP channel of vascular tissue,25 and H2S could also inhibit vascular SMC proliferation,26 and CSE mRNA was expressed mainly by the SMC of the vascular cells but not by the endothelial cells.27 A potentially critical role for CSE-derived H2S in allergic response is its ability to regulate airway smooth muscle associated with the tone of vascular. H2S can also suppress leukocyte adherence to the vascular endothelium and reduce leukocyte infiltration and oedema formation.28 We speculated that the down-regulation of the H2S/CSE pathway in the nasal mucosa of AR was associated with the change of blood flow. The release of H2S was decreased as CSE was suppressed by PPG leading to increased vasodilatation, and increased permeability of the nasal mucosa epithelium and vascular endothelium, and rapidly generated mediators of inflammation (leukotrienes, chemokines), associated with the allergic response, including rhinorrhoea, mucosal oedema, neutrophil and eosinophil chemotactic effects. The level of H2S increased by NaHS had the opposite results with vasoconstrictor effects and was accompanied by the inhibition of inflammatory mediator release.
As mentioned above, H2S is also an important modulator of vascular tone like NO and CO, and plays an anti-oxidant role in inflammation.29 It should be noted that NO and CO may have direct vasorelaxation through endothelial cells.30 Immunohistochemistry researches show that NOS and HO, which are major rate-limited enzymes of NO and CO respectively, are mainly located in cytoplasm of endothelial cells.31 But the expression of H2S-generating enzyme was identified in vascular SMC, not in endothelium27 and it was shown that the vasorelaxant effect of H2S might mainly mediate by an interaction of the gas with smooth muscles.24 Thus, H2S seems to have a unique action mechanism among vasodilator gases in nasal mucosa. The gas signals among NO, CO and H2S may be a self-balancing regulation of endothelial cells and muscle cells in nasal mucosa of allergic inflammation. Vasorelaxation of H2S may be influenced by NO or CO, a recent study also suggests that low doses of H2S may induce vasoconstriction by scavenging endothelial NO.32,33 Perhaps the roles of H2S are regulated by NO or CO in the pathogenesis of AR, although further research is needed.
ConclusionsOur findings have shown that down-regulation of endogenous H2S pathway in AR, and the level of H2S was positively correlated with the expression of CSE in nasal mucosa. It indicated that endogenous H2S had latent roles in the pathogenesis of AR. Furthermore, the roles of H2S in pathogenesis of AR appear so complex, and more new lines of research are needed, and might have potential benefit for the investigation of AR.
This study was supported by the National Science Foundation of China (No. 30400494) and National Science Foundation of Shandong province (No. Y2006C03).