Asthma is an inflammatory disease of the lower airways characterised by the presence of airway inflammation, reversible airflow obstruction and airway hyperresponsiveness and alterations on the normal structure of the airways, known as remodelling. Remodelling is characterised by the presence of metaplasia of mucous glands, thickening of the lamina reticularis, increased angiogenesis, subepithelial fibrosis and smooth muscle hypertrophy/hyperplasia. Several techniques are being optimised at present to achieve a suitable diagnosis for remodelling.
Diagnostic tools could be divided into two groups, namely invasive and non-invasive methods. Invasive techniques bring us information about bronchial structural alterations, obtaining this information directly from pathological tissue, and permit measure histological modification placed in bronchi layers as well as inflammatory and fibrotic cell infiltration. Non-invasive techniques were developed to reduce invasive methods disadvantages and measure airway remodelling-related markers such as cytokines, inflammatory mediators and others.
An exhaustive review of diagnostic tools used to analyse airway remodelling in asthma, including the most useful and usually employed methods, as well as the principal advantages and disadvantages of each of them, bring us concrete and summarised information about all techniques used to evaluate alterations on the structure of the airways. A deep knowledge of these diagnostic tools will make an early diagnosis of airway remodelling possible and, probably, early diagnosis will play an important role in the near future of asthma.
Asthma is an inflammatory disease of lower airways characterised by the presence of airway inflammation, reversible airflow obstruction and airway hyperresponsiveness and, as lately described, alterations on the normal structure of the airways.1 The presence of these alterations, known as remodelling, has been postulated to play an important role in the pathogenesis of asthma, and the absence of treatment of these alterations may be a lack in the prognosis of asthma patients. Remodelling is characterised by the presence of metaplasia of mucous glands, thickening of the lamina reticularis, increased angiogenesis, subepithelial fibrosis and smooth muscle hypertrophy/hyperplasia.2
Several immune cells have been related with the pathogenesis of airway remodelling, including T cells, eosinophils, mast cells, fibroblasts and other epithelial cells.3 These cells could be evaluated by different invasive methods (including bronchial biopsies, analysis of bronchoalveolar lavage solid phase, etc.) or non-invasive methods (i.e. induced sputum solid phase analysis). Pathogenesis of airway remodelling is also related with structural alterations of the airway,4 which could be diagnosed by imaging techniques (i.e. high resolution computerised tomography) and invasive methods such as bronchial biopsies. The presence of different pro-inflammatory mediators and cytokines involved in remodelling3 could be evaluated through invasive methods (e.g. detecting mediators in bronchial biopsies or bronchoalveolar lavage), as well as indirect methods (including analysis of induced sputum liquid phase). Finally, asthma has always been related with functional alterations, evaluation of bronchial hyperresponsiveness and lung function test could bring us important information about the level of bronchial remodelling.
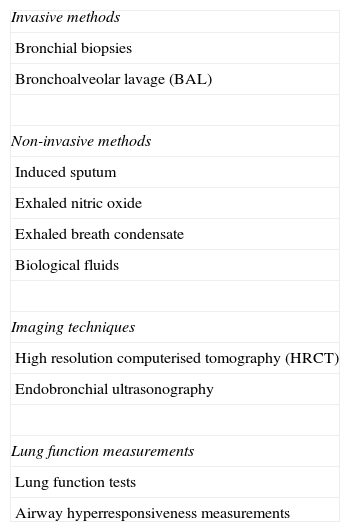
Early diagnosis of airway remodelling will play an important role in the near future of asthma; several techniques are being optimised at present to achieve a suitable diagnosis. All these techniques are summarised in Table 1.
Diagnostic tools employed to assess airway remodelling.
| Invasive methods |
| Bronchial biopsies |
| Bronchoalveolar lavage (BAL) |
| Non-invasive methods |
| Induced sputum |
| Exhaled nitric oxide |
| Exhaled breath condensate |
| Biological fluids |
| Imaging techniques |
| High resolution computerised tomography (HRCT) |
| Endobronchial ultrasonography |
| Lung function measurements |
| Lung function tests |
| Airway hyperresponsiveness measurements |
Remodelling is characterised, as previously described, by the presence of different structural alterations such as metaplasia of mucous glands, thickening of the lamina reticularis, increased angiogenesis, subepithelial fibrosis and smooth muscle hypertrophy/hyperplasia.2 All these structural modifications are caused by different immunological-related mediators or cellular mechanisms. Structural alterations are accompanied by functional changes, and both sorts of changes are obviously related. Regarding these aspects it is essential to introduce the epithelial mesenchymal trophic unit (EMTU) concept, described by Plopper and Evans.5 The EMTU is a concept linked to the existence of an attenuated fibroblast layer between epithelial and mesenchymal cells. These fibroblasts are able to differentiate into myofibroblasts, as a response to some local stimuli, and secrete different cellular mediators and proteins known as extracellular matrix (ECM). ECM is a dynamic structure that influences epithelium cells, smooth muscle cells, blood vessels and neural terminations. Activated EMTU is related with the persistence of airway inflammation, the abnormal reparation of damaged epithelium and fibroblasts proliferation, creating a microenvironment similar to a chronic wound in the asthmatic airway. In short, EMTU is referred to different airway structures, cells layers and secreted mediators that control airway inflammation and repair process.
It is essential to understand how EMTU controls the different processes involving airway structure, and the important role that ECM plays in airway remodelling. After an acute epithelial injury (i.e. tobacco smoke or allergen exposure, viruses), an increment in the number of fibroblasts occurs, accompanied by an increase in the ECM production (including proteins like fibronectin, tenascin and I–III–V collagen) that alters smooth muscle function.6 All these changes are regulated by several cytokines and pro-fibrotic mediators, such as transforming growth factor (TGF)-β and epidermal growth factor (EGF) mainly, resulting in an increment of subepithelial fibrosis. Simultaneously, increased angiogenesis and vascular permeability promoted by vascular endothelial growth factors (VEGFs) secreted by smooth muscle cells,7 induce airway oedema and inflammatory cells recruitment (mainly eosinophils and mast cells). All these changes induce smooth muscle and epithelial cell proliferation, with an increased production of different cytokines like interleukin 1 (IL-1), IL-5, IL-13 or tumour necrosis factor-α (TNF-α), lipidic mediators like leukotriene D4 (LTD4) or prostaglandine E2 (PGE2) and growth factors like EGF, TGF-β, platelet derived growth factors (PDGFs) or VEGFs (Tables 2 and 3). Production of all these mediators provides a source of chronic inflammation and fibrosis that causes all the structural alterations mentioned above, leading the asthmatic airway to a remodelling process.
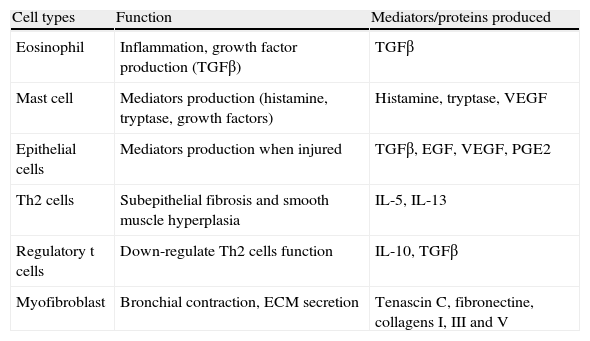
Most relevant cells involved in bronchial remodelling.
| Cell types | Function | Mediators/proteins produced |
| Eosinophil | Inflammation, growth factor production (TGFβ) | TGFβ |
| Mast cell | Mediators production (histamine, tryptase, growth factors) | Histamine, tryptase, VEGF |
| Epithelial cells | Mediators production when injured | TGFβ, EGF, VEGF, PGE2 |
| Th2 cells | Subepithelial fibrosis and smooth muscle hyperplasia | IL-5, IL-13 |
| Regulatory t cells | Down-regulate Th2 cells function | IL-10, TGFβ |
| Myofibroblast | Bronchial contraction, ECM secretion | Tenascin C, fibronectine, collagens I, III and V |
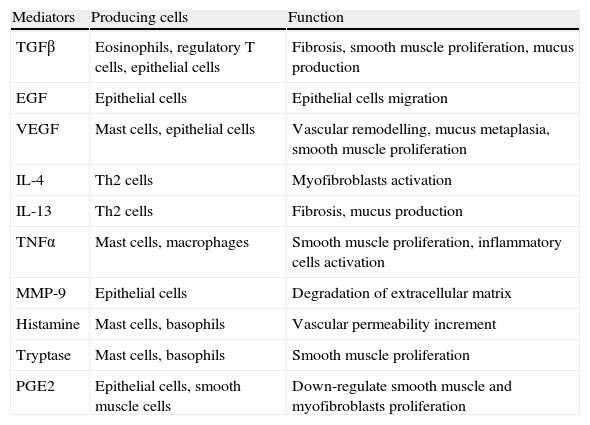
Most relevant mediators involved in bronchial remodelling.
| Mediators | Producing cells | Function |
| TGFβ | Eosinophils, regulatory T cells, epithelial cells | Fibrosis, smooth muscle proliferation, mucus production |
| EGF | Epithelial cells | Epithelial cells migration |
| VEGF | Mast cells, epithelial cells | Vascular remodelling, mucus metaplasia, smooth muscle proliferation |
| IL-4 | Th2 cells | Myofibroblasts activation |
| IL-13 | Th2 cells | Fibrosis, mucus production |
| TNFα | Mast cells, macrophages | Smooth muscle proliferation, inflammatory cells activation |
| MMP-9 | Epithelial cells | Degradation of extracellular matrix |
| Histamine | Mast cells, basophils | Vascular permeability increment |
| Tryptase | Mast cells, basophils | Smooth muscle proliferation |
| PGE2 | Epithelial cells, smooth muscle cells | Down-regulate smooth muscle and myofibroblasts proliferation |
MMP-9 (matrix metalloproteinase-9).
Several years ago specimens obtained from post-mortem studies brought us the first information about structural alterations in asthmatic lungs. Invasive methods of analysing bronchial morphology are able to quantify remodelling alterations in a direct way, showing structural modifications and bronchial wall cell infiltration present in bronchial specimens. These methods allow evaluating inflammatory and epithelial cells, as well as cytokines, inflammatory and pro-fibrotic mediators that are present in subjects in different moments of their process. Invasive methods are usually employed in investigational studies and have been used to evaluate evolution of asthma and response to treatment. The most commonly employed invasive methods, including bronchial biopsies and bronchoalveolar lavage, will be described below.
Bronchial biopsiesBronchial biopsy (BB) was first employed to evaluate asthma severity in the early 1960s, but it was in the 1990s when BB became extensively used to assess airway remodelling in asthmatic patients.8 BB allows evaluating structural and morphological changes in bronchial specimens with the lack of analysing not the entire airway wall, but superficial airway layers. At least, five to eight specimens should be obtained for a correct diagnosis.9 Two different methods for obtaining BB have been usually employed: endobronchial biopsy (EBB) and transbronchial biopsy (TBB). Both techniques should be performed by specialists in a hospital environment using flexible bronchoscopy. EBB makes it possible to obtain large airway specimens, while TBB permits to obtain distal airway and alveolar tissue specimens. EBB presents fewer complications than TBB, but information regarding airway remodelling is more extensive when TBB is employed, this is due to the capability of analysing distal airway remodelling.10 Both biopsy techniques have been shown to give similar results when compared with surgically obtained specimens.11
BB has been employed in several studies to assess airway remodelling, some of them employing EBB12–14 and another TBB,15 and some studies have even employed and compared both techniques.16,17 Most of these studies have analysed the presence of inflammatory cells, fibroblasts and inflammatory mediators in bronchial specimens, but BB has also been employed to elucidate asthmatic patients’ response to corticosteroid treatment.18–20 BB obtained before and after corticosteroid treatment gives important information about the airway structural modifications produced by these drugs. The use of BB has been implemented in the past years, regarding different aspects of asthma pathogenesis, and BB has been employed, for example, to evaluate the relationship between airway remodelling and airway hyperresponsiveness21 or between airway remodelling and inflammation.22
In short, BB has probably become the most useful technique to evaluate airway remodelling and its relationship with other aspects of asthma pathology, such as airway inflammation or treatment response. Even though BB is undoubtedly useful, it also presents some disadvantages such as the risk of severe complications (like bleeding or pneumothorax); the difficulty to obtain suitable specimens; or the requirement of trained personal to perform flexible bronchoscopy.
Bronchoalveolar lavageBronchoalveolar lavage (BAL) could be considered a complementary tool of bronchial biopsies, since both kinds of samples should be obtained by bronchoscopy techniques. BAL could be obtained in the same intervention as bronchial biopsies, and could give us important information about cells and airway remodelling-related markers, without biopsy-associated risks. The BAL technique consists in instilling saline solution, through the instrumentation channel of a bronchoscope, into a segmental or subsegmental bronchus. After saline instilling, this solution is then aspirated into a sterile container. Specimens obtained should be processed and centrifuged to obtain a solid and a liquid phase.
As well as biopsy, BAL makes it possible to evaluate inflammatory cells related to airway remodelling in BAL solid phase obtained after centrifugation. Different articles23–25 have shown elevated levels of T regulatory cells, fibrocytes and neutrophils in BAL from asthmatic subjects, and its relationship with airway inflammation and remodelling. But, BAL has also been employed to analyse remodelling-related markers, for these studies the liquid phase of BAL was obtained after centrifugation. Different markers, such as transforming growth factor-β1 (TGF-β1) or insulin-like growth factor binding protein-3 (IGFBP3) presented modified levels in the liquid phase of BAL, when compared with control subjects’ ones.26,27
In resume, BAL presents advantages like the possibility of obtaining samples simultaneously to bronchial biopsies, evaluating inflammatory cells or remodelling-related mediators, and evaluation simplicity. On the other hand, BAL disadvantages lies in the difficulty to obtain samples (bronchoscopy is required) and the limited amount of epithelium and epithelial underlying layers cells obtained.
Non-invasive methodsIn the last 50 years clinical practice has tried to reduce the use of invasive techniques for diagnosing airway pathologies. Technical and clinical difficulties involving direct or invasive methods have promoted the development of different non-invasive methods to evaluate the presence of airway remodelling in asthmatic patients. Non-invasive methods are based in the study of different inflammatory cells and mediators related with airway remodelling obtained from sputum, bronchoalveolar lavage and other systemic fluids, or from breath exhaled. Non-invasive methods bring information about clinical features as well as investigational data of evolution and modification of asthmatic disease.
Induced sputumInduced sputum (IS) first protocol was described by Bickerman in 195828; after this description, several studies have demonstrated the usefulness of IS when diagnosing different respiratory diseases.29 Sputum induction consists in the inhalation of increasing concentrations of hypertonic saline (3%, 4% and 5%) during 10min, and obtaining the sputum produced in a sterile container. Solid sputum material is separated from saliva, treated with 0.1% dithiothreitol (DTT) and mucus is removed by filtration. Material obtained is centrifuged to separate liquid and solid phase.30 IS analysis, after processing and centrifugation of samples, permits to evaluate levels of inflammatory cells, lipidic mediators and cytokines in a safe way, even in moderate or severe asthma.31
Flow cytometry or cytospin analysis and microscopical cell count, performed in solid phase of IS, make it possible for investigators to establish a cellular pattern involving respiratory pathologies, including asthma airway remodelling. For example, Kaminska et al.32 tried to identify the inflammatory cell patterns of airway remodelling in different subtypes of severe asthma; eosinophils count performed in IS showed to not be able to identify such different subtypes. Eosinophils count in IS, together with exhaled nitric oxide and remodelling-related markers like interleukins, has also been employed in children to identify two different phenotypes of moderate asthma.33 Recently, Broekema et al.34 employed IS to evaluate differences in airway remodelling between actually asthmatic patients and patients with clinical or complete asthma remission. Finally, IS solid phase has also been demonstrated to be useful when evaluating airway remodelling response to corticosteroid treatment in asthmatic patients.35
The liquid phase of IS has been employed to evaluate levels of pro-inflammatory markers and cytokines involving airway remodelling. This is probably the most known featuring of IS, and it has been defined as a relevant technique when evaluating different remodelling markers such as TGF-β1, vascular endothelial growth factor (VEGF), matrix metalloproteinase-9 (MMP-9) and others.36–38 But analysis of the liquid phase of IS, has also been demonstrated as a useful tool to evaluate airway remodelling differences between different coughing pathologies, like asthma and eosinophilic bronchitis.39
In summary, due to its different applications; the fact that it is easy to obtain; and the absence of risk for patients, IS has become, probably, the most useful technique to evaluate airway remodelling in a non-invasive manner.
Exhaled nitric oxideExhaled nitric oxide (FeNO) has been related with the presence of inflammation in bronchi of asthmatic patients,40 and it has been also proposed as an efficient technique to evaluate asthmatic exacerbations41 and asthmatic response to treatment.42 The method of FeNO measuring is simple and is performed by breathing through a chemiluminescence analyser for about 6s, according to the American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines.
Several studies have suggested that FeNO could represent an easy and accessible tool to assess airway remodelling in adults as well as in children suffering asthma. Ketai et al.43 demonstrated that FeNO levels were elevated during acute asthma exacerbations, related with bronchial wall area assessed by high-resolution computed tomography, but these elevated levels did not persist when acute exacerbations were treated. When the FeNO and airway remodelling relationship has been measured in children44 a positive correlation with FeNO has been demonstrated. This correlation has also been shown when adolescents were evaluated,45 and elevated levels of FeNO were related with AHR and airway remodelling in children ≥12 years of age.
Although FeNO is well related with bronchial inflammation and seems to be linked to airway remodelling, this last relationship still remains unclear. Most studies that have been performed evaluating FeNO levels present a lack of dispersion referring to age of patients, severity of asthma and methods used to evaluate remodelling. More specific studies, especially regarding severity of asthma, are necessary to assure that FeNO is a useful tool to evaluate airway remodelling.
Exhaled breath condensateNot many articles regarding the use of exhaled breath condensate (EBC) in the study of airway remodelling have been published. Despite the recent use of EBC to establish the existence of airway remodelling in asthmatic patients, it seems to be a useful tool. To obtain EBC, a refrigerating exhaled breath circuit should be used. Patients have to breathe through this circuit at tidal volume for 10min and samples obtained should be processed before analysing.
Lex et al.46 demonstrated the relationship between levels of cystenyl leukotrienes (cysLTs), previously described as a marker for airway remodelling,47 measured in EBC, and airway remodelling, assessed as reticular basement membrane thickening in biopsies obtained in children. Another study has described the relationship between Endotelin-1 (ET-1), another marker implicated in airway remodelling, measured in EBC, with different degrees of asthma severity in adult asthmatic patients.48
Evaluation of EBC levels of remodelling-related markers has been demonstrated as a valid technique when evaluating remodelling, although more studies are necessary to assure this point.
Peripheral blood, urine, saliva and other biological fluidsThe analysis of airway remodelling-related mediators in different peripheral fluids seems to be an economical and easy way to evaluate airway remodelling levels in asthmatic patients. Peripheral fluids have been employed in murine models to evaluate these remodelling markers and, in the past years, have been developed in humans. MMP-9 levels in asthmatic patients’ blood have been evaluated in different studies,49,50 showing contradictory results. Recently, another study performed in humans,51 has evaluated levels of Fibulin-1 (a secreted glycoprotein that assists in stabilising extracellular matrix) in serum of asthmatic patients and healthy volunteers; Fibulin-1 levels were significantly increased in asthmatic patients.
Further studies are necessary, involving different peripheral fluids, cells and related markers, to establish the relevance of these techniques when evaluating airway remodelling.
Imaging techniquesRecent advances in imaging techniques provide asthma with a new course to evaluate the presence of remodelling in bronchial airway. Improvement in computerised tomography (CT) resolution, the employing of new collimation techniques and new reconstruction algorithms bring imaging techniques a leading role in asthma evaluation. Moreover, improvements in endoscopic ultrasonography techniques, including smaller probes and better resolution images, complete the advances that make the indirect evaluation of airway remodelling through imaging techniques possible.
High resolution computerised tomographyBronchial wall thickening has been described as one of the most important findings in asthma patients but not in healthy subjects,52 but its evaluation required obtaining bronchial biopsies. To solve the lack of obtaining bronchial specimens, computerised tomography (CT) and especially high resolution CT (HRCT) have been employed in several trials as a bloodless method to evaluate bronchial airway structural changes. In contrast, HRCT presents high radiation exposition and an elevated cost as principal objections to its extended use. Due to these disadvantages, at present the CT technique is limited to investigational trials and severe asthma patients.
Several different methods have been employed to evaluate bronchial wall thickening, including manual or computer-based detection of bronchi,53 evaluation of different amounts of bronchi54 or, even, three-dimensional evaluation of lung structure.55 All these methods have been proved as valid, to assess airway remodelling56,57 and have been compared with histological techniques.58 Finally, HRCT has been employed to evaluate the relationship between inflammation and airway remodelling59 and to assess the presence of remodelling in asthmatic children54 with encouraging results.
Endobronchial ultrasonographyEndobronchial ultrasonography (EBUS) was described in the early 1990s by Hurter et al.60 Its first use was to evaluate the infiltration of different tumours in bronchial wall, and the presence of lymph node infiltration. EBUS is based in ultrasound technique; a probe surrounded by a saline-filled balloon is introduced in bronchi through a bronchoscope. Ultrasounds have sufficient penetration to evaluate the whole bronchial wall (about 2cm) and provide the optimum resolution image.61,62
EBUS has been demonstrated to be able to distinguish three to five layers of the bronchial wall62 and was first employed to assess bronchial wall thickness by Shaw et al. in 2004.63 Recent studies, like that published by Soja et al.,64 demonstrated the utility of EBUS to measure bronchial wall layers in asthmatic patients, showing no discrepancies with results obtained by HRCT, and the usefulness of the measure of wall area and wall area/total diameter ratio. Published studies have demonstrated neither alterations of bronchial diameter nor wall thickness63 related to ultrasound probe bronchial introduction.
According to all these reasons and the absence of radiation, EBUS could play an important role in the diagnosis of airway remodelling in asthma patients.
Lung function measurementsDiagnostic tools described previously demonstrated the presence of structural alterations in asthmatic airway. But these structural modifications are accompanied by clinical alterations, responsible for clinical worsening and symptoms. Lung function measurements, combined with structural alteration diagnostic techniques, make it possible to evaluate the asthmatic process in depth. Alterations in lung function tests and airway hyperresponsiveness are the most used tools to evaluate lung modifications among asthmatic patients.
Lung function testsFollow-up studies have demonstrated the decline of lung function assessed by bronchial function test, and mainly expressed as forced expiratory volume in 1s (FEV1), in asthmatic patients.65 Lung function decline has been proposed to be related with the presence of remodelling, based on the increment of lung function after corticosteroid treatment in asthma patients.66 According to these theories, bronchial function test has been proposed to be an important tool to explore airway remodelling in asthma. Different studies67–69 have suggested that decline of lung function (or even AHR as is next described) is related with airway remodelling, expressed as reticular basement membrane thickening.
Airway hyperresponsiveness measurementsThe link between airway hyperresponsiveness (AHR) and remodelling is not well defined, and several studies regarding this theory have been published. Results obtained from different authors seem to be contradictory. Whereas the first studies published suggested the existence of a relationship between AHR and an increment in airway remodelling, recent studies suggest that this relationship is not clear. AHR measurement is performed by the inhalation at tidal volume of a bronchoconstrictor agent (methacholine, histamine, manitol, etc.), using a continuous pressurised nebuliser, at different concentrations. Concentration of the bronchoconstrictor agent causing 20% or 15% fall, depending on the agent, in FEV1, named PC20 or PC15, is employed to express test results. Presence of AHR is considered when PC20 is lower than a previously established value for each agent.70
Morphometric in vitro models, such as that proposed by Heather L. Gillis et al.71 in 1999, demonstrated a connection between airway remodelling, airway smooth muscle and airway hyperresponsiveness (AHR). Laprise et al.72 also reached the same conclusion in a follow-up study that included subjects with AHR but without asthma symptoms; some patients developed asthma symptoms and all of them presented an increment of remodelling markers and subepithelial fibrosis in bronchial biopsies. A study developed by Kariyawasam et al.73 including 30 atopic patients with asthma symptoms and AHR defined as methacholine PC20 of 8mg/ml or less, or a FEV1 increment higher than 15% to β2-agonist, supported these results. Allergen challenge test was performed and airway remodelling was assessed by bronchoscopy with bronchial biopsies at baseline, 24h and seven days after challenge test. Inflammation-related mediators were also evaluated. Results showed that remodelling-related mediators persisted elevated seven days, whereas inflammation related mediators decreased in the first 24h after challenge tests. These results suggest that remodelling is related with AHR increment after exposition to allergen in asthmatic patients.
On the other hand, studies like that performed by Siddiqui et al.74 in 2008 including asthmatic and non-asthmatic eosinophilic bronchitis (EB) patients showed no relation between AHR and airway remodelling. There were no differences in remodelling structural changes (defined as an increment in ASM or reticular basement membrane thickening) assessed by bronchial biopsy, between asthmatic patients (that presented AHR) and EB patients (without AHR), although both groups of patients showed differences when compared with a healthy control group.
The relationship between AHR and airway remodelling could play an important role when evaluating the presence of structural alterations in the airway. But discrepancies between studies make new studies to assess this relationship necessary.
ConclusionsAsthma airway remodelling has become an important way of evaluating asthma severity, asthmatic response to treatment and lung function decline. Since its description as an asthma characteristic, airway remodelling has been evaluated in many different ways, including invasive and non-invasive techniques.
Invasive techniques bring us information about bronchial structural alterations, obtaining this information directly from pathological tissue. Invasive techniques permit us to measure histological modification placed in bronchi layers, as well as inflammatory and fibrotic cell infiltration. Invasive methods were the first tools that made airway remodelling measurement possible, and play, undoubtedly, a remarkable role in its evaluation. Unfortunately, invasive methods (principally BB and BAL) present some disadvantages such as the need for specially trained personal and hospital environment, risks for patients and elevated costs.
Non-invasive techniques were developed to reduce the disadvantages of invasive methods. Non-invasive techniques measure airway remodelling-related markers, such as cytokines, inflammatory mediators and others. These methods are based on the evaluation of exhaled breath (FeNO and EBC), sputum (IS), lung function (pulmonary function test and AHR measurement) or lung images (CT and EBUS). The risk associated to invasive techniques has been avoided, and in most of these non-invasive methods, no trained personal is needed and they should not be performed in a hospital environment. For example, FeNO is an inexpensive technique that could be carried out in an outpatient clinic and without risks for the patient. But unfortunately, non-invasive techniques present other disadvantages like radiation exposition and elevated cost (referring to HRCT), the need for expensive laboratory equipment (especially in IS and EBC technique) and, sometimes, they are not free of risks for the patients (i.e. EBUS).
In conclusion, methods assessing airway remodelling in asthma have been greatly improved in the last years, making them more secure, reliable and generalised than before. Nevertheless, the relevance of these techniques still remains unclear and further studies are necessary to elucidate if it is of worth to perform some of them in routine clinic.
FundingAuthors do not report any funding for this article.
Conflict of interestThe authors declare not to have any conflict of interest.