Bronchial asthma is characterised by airway inflammation and remodelling with a decline of lung function. Fibrocytes are bone marrow-derived mesenchymal progenitor cells that play important roles in the pathogenesis of airway remodelling. Several clinical parameters are currently being used in routine clinical practice to assess outcome of therapy in asthma including frequency of rescue with short-acting β2-agonist and the asthma control test. In this study, we hypothesised that asthma control test is associated with circulating levels of fibrocytes in bronchial asthma.
MethodsThere were 20 patients with asthma and seven healthy controls. The number of CD45+Collagen I+ circulating fibrocytes was assessed in the peripheral blood by flow cytometry.
ResultsThe number of circulating fibrocytes was significantly increased in asthma patients with moderate and severe disease compared to controls, and it was inversely correlated with % forced expiratory volume in one second and % forced vital capacity (%FVC). The frequency of inhalation of short-acting β2 agonist and the asthma control test score was significantly and inversely correlated with the number of circulating fibrocytes.
ConclusionThe results of this study showed that the number of circulating fibrocytes is inversely correlated with clinical asthma control parameters, further supporting the relevance of measuring circulating fibrocytes as a marker of clinical control in bronchial asthma.
The airway of patients with long-term bronchial asthma is characterised by chronic inflammation and structural tissue remodelling.1–3 Structural remodelling in asthmatic airways includes epithelial detachment, increased airway smooth muscle mass, mucous gland/goblet cell hyperplasia, proliferation of blood vessels, and subepithelial fibrosis.4 Subepithelial fibrosis is characterised by extensive deposition of extracellular matrix (ECM) components leading to a decline in lung function and irreversible airway obstruction.5 One of the mechanisms of the enhanced ECM deposition is the accumulation of activated fibroblasts and myofibroblasts.5 In particular, myofibroblasts are the main players in the pathogenesis of airway wall fibrosis because they produce and secrete collagens and other ECM molecules.4 The origin of myofibroblasts may be multiple; some of them derive from pre-existing fibroblasts or from airway smooth muscle cells that migrate towards the epithelial basement membrane and differentiate into myofibroblasts under the stimulation of cytokines and growth factors, whereas others originate from fibrocytes that are bone marrow-derived mesenchymal progenitor cells that coexpress markers of haematopoietic and stromal cells and differentiate to fibroblasts and myofibroblasts by transforming growth factor-β1 or endothelin-1.6–8 Several lines of evidence have shown that fibrocytes significantly accumulate in the asthmatic airway wall not only in patients with progressive, chronic obstructive, untractable and severe asthma but also in patients in early stages of the disease, suggesting their role in the onset of airway tissue remodelling.9–14 The mainstay of current asthma therapy is the use of inhaled corticosteroids alone or in combination with long- or short- (SABA) acting β2-agonists.15 Several parameters have been suggested to use for assessing the outcome of therapy in asthma including signs of clinical impairment (symptom frequency, the rescue with SABA, sleep interference) and scoring systems such as the Asthma Control Questionnaire (ACQ), the Asthma Control Test (ACT)) or the Test for Respiratory and Asthma Control in Kids (TRACK).16 These asthma control composite tools are being extensively used because of the importance of therapeutic control in asthma.17–19 Among all parameters, ACT has the largest number of validation data.16
No previous study has explored whether circulating fibrocytes are related to asthma control composite scores. In the present investigation, we tested the hypothesis that the number of circulating fibrocytes is correlated with the ACT score in patients with chronic bronchial asthma.
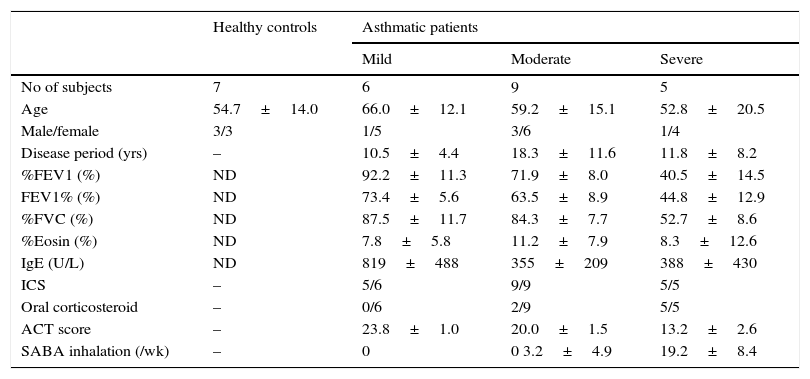
Materials and methodsPatients and measurementsThis study comprised 20 stable asthmatic patients that consulted at Mie University Hospital. Among patients, six had mild, nine moderate, and five had severe asthma but without clinical exacerbation. The diagnosis of the disease stage was based on the Guidelines of Japanese Society of Allergology and Global Initiative for Asthma (GINA).20,21 All patients were being treated with standard doses of inhaled corticosteroids and relievers (short-acting beta-2-agonist) on demand before consultation. Clinical data and pulmonary function tests were obtained from case records. Blood samples for evaluating circulating fibrocytes were obtained from seven healthy controls and from 20 patients on the first day of consultation after obtaining informed consent (Table 1). To score the asthma clinical control on the date of consultation, all patients were asked to complete the ACT questionnaire translated to Japanese language. The research protocol was approved by the Ethics Committee for Clinical Investigation of Mie University, Japan.
Characteristics of the subjects.
| Healthy controls | Asthmatic patients | |||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| No of subjects | 7 | 6 | 9 | 5 |
| Age | 54.7±14.0 | 66.0±12.1 | 59.2±15.1 | 52.8±20.5 |
| Male/female | 3/3 | 1/5 | 3/6 | 1/4 |
| Disease period (yrs) | – | 10.5±4.4 | 18.3±11.6 | 11.8±8.2 |
| %FEV1 (%) | ND | 92.2±11.3 | 71.9±8.0 | 40.5±14.5 |
| FEV1% (%) | ND | 73.4±5.6 | 63.5±8.9 | 44.8±12.9 |
| %FVC (%) | ND | 87.5±11.7 | 84.3±7.7 | 52.7±8.6 |
| %Eosin (%) | ND | 7.8±5.8 | 11.2±7.9 | 8.3±12.6 |
| IgE (U/L) | ND | 819±488 | 355±209 | 388±430 |
| ICS | – | 5/6 | 9/9 | 5/5 |
| Oral corticosteroid | – | 0/6 | 2/9 | 5/5 |
| ACT score | – | 23.8±1.0 | 20.0±1.5 | 13.2±2.6 |
| SABA inhalation (/wk) | – | 0 | 0 3.2±4.9 | 19.2±8.4 |
Circulating fibrocytes were identified as previously described by flow cytometry in blood sampled during the first visit.22,23 Briefly, after freshly isolated blood was cultured in sodium chloride lysis solution, leukocytes were stained with anti-CD45-PerCP (BD Biosciences), and after permeabilisation using cytofix/cytoperm (BD Biosciences), intracellular staining of collagen was performed with unconjugated rabbit anti-human Col-I antibody followed by staining with Alexa-Fluor 568 goat anti-rabbit. Samples were then processed on a FACS Calibur flow cytometer using Cellquest software. CD45+Col-I+ leukocytes were counted as fibrocytes. It is worth noting that some limitations have been reported about this method of fibrocyte counting.24–26
Statistical analysisThe collected data were analysed using StatView statistical package (Abacus Concepts, Berkeley, CA, USA). Statistical differences were evaluated by analysis of variance (ANOVA) with post hoc analysis using the Fisher's predicted least significant difference test. Results were considered statistically significant if p<0.05.
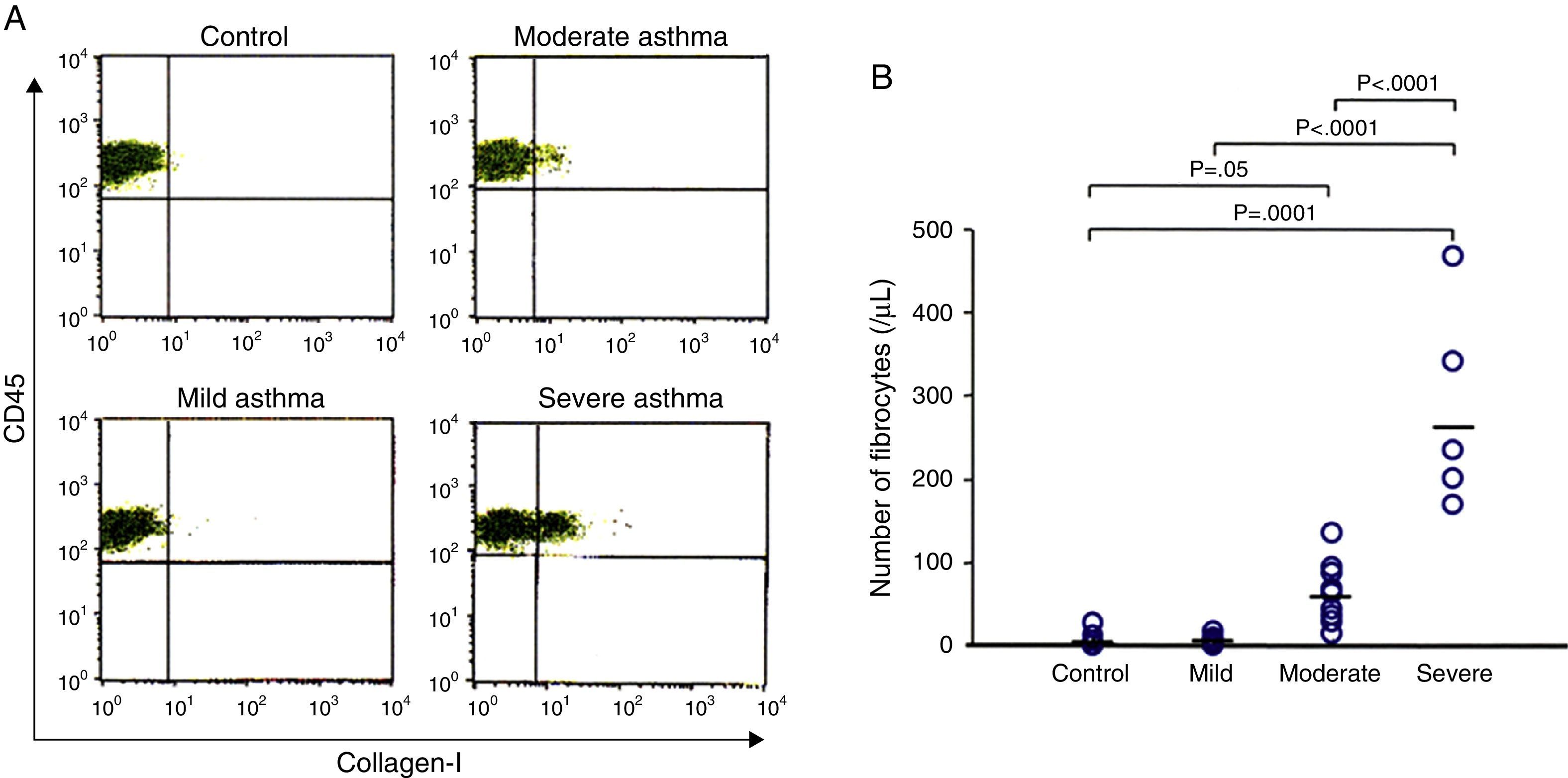
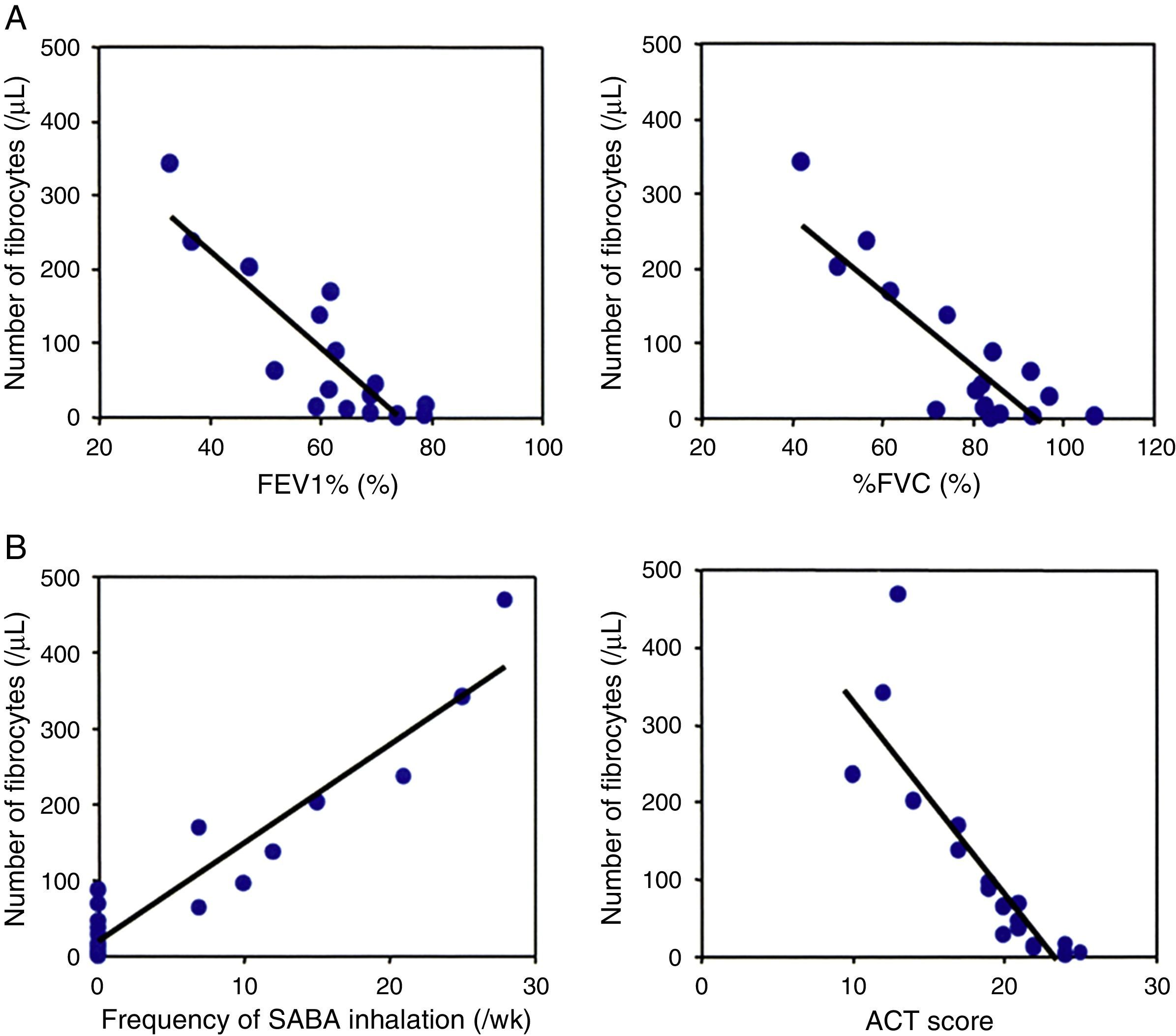
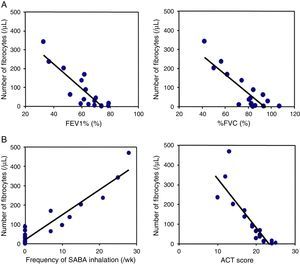
ResultsNumber of circulating fibrocytes and correlation with lung functionThe number of fibrocytes in peripheral blood was evaluated by disease severity in asthmatic patients and healthy controls. The number of fibrocytes was significantly increased in patients with asthma proportionally to the severity of disease (Fig. 1A and B). Pulmonary function test was performed in all asthmatic patients except two patients that had a cough attack during the examination. The number of circulating fibrocytes was inversely correlated with the % forced expiratory volume in one second (FEV1%, r=−0.8, p<0.05) and with the % forced vital capacity (%FVC, r=−0.8, p<0.05) (Fig. 2A). The number of circulating fibrocytes was not significantly correlated with the %V50 and %V25 (data not shown).
Number of circulating fibrocytes in asthmatic patients. Fibrocytes defined as CD45+Collagen-I+ cells were identified by using flow cytometry (A). The number of fibrocytes in controls (6.4±10.2/μL), and in mild (6.6±6.0/μL), moderate (63.5±38.1/μL) and severe (283.2±122.1/μL) asthmatic patients were measured. The number of fibrocytes was significantly higher in patients with much more severe disease than in those with less severe asthma and healthy controls (B).
The relation of the number of fibrocytes with lung functional parameters and ACT. The number of circulating fibrocytes was inversely correlated with % forced expiratory volume in one second (FEV1%, r=−0.8, p<0.05) and with % forced vital capacity (%FVC, r=−0.8, p<0.05) (A). The number of circulating fibrocytes was significantly correlated with the frequency of SABA inhalation (r=0.9, p<0.05) and inversely correlated with the ACT scores (r=−0.8, p<0.05).
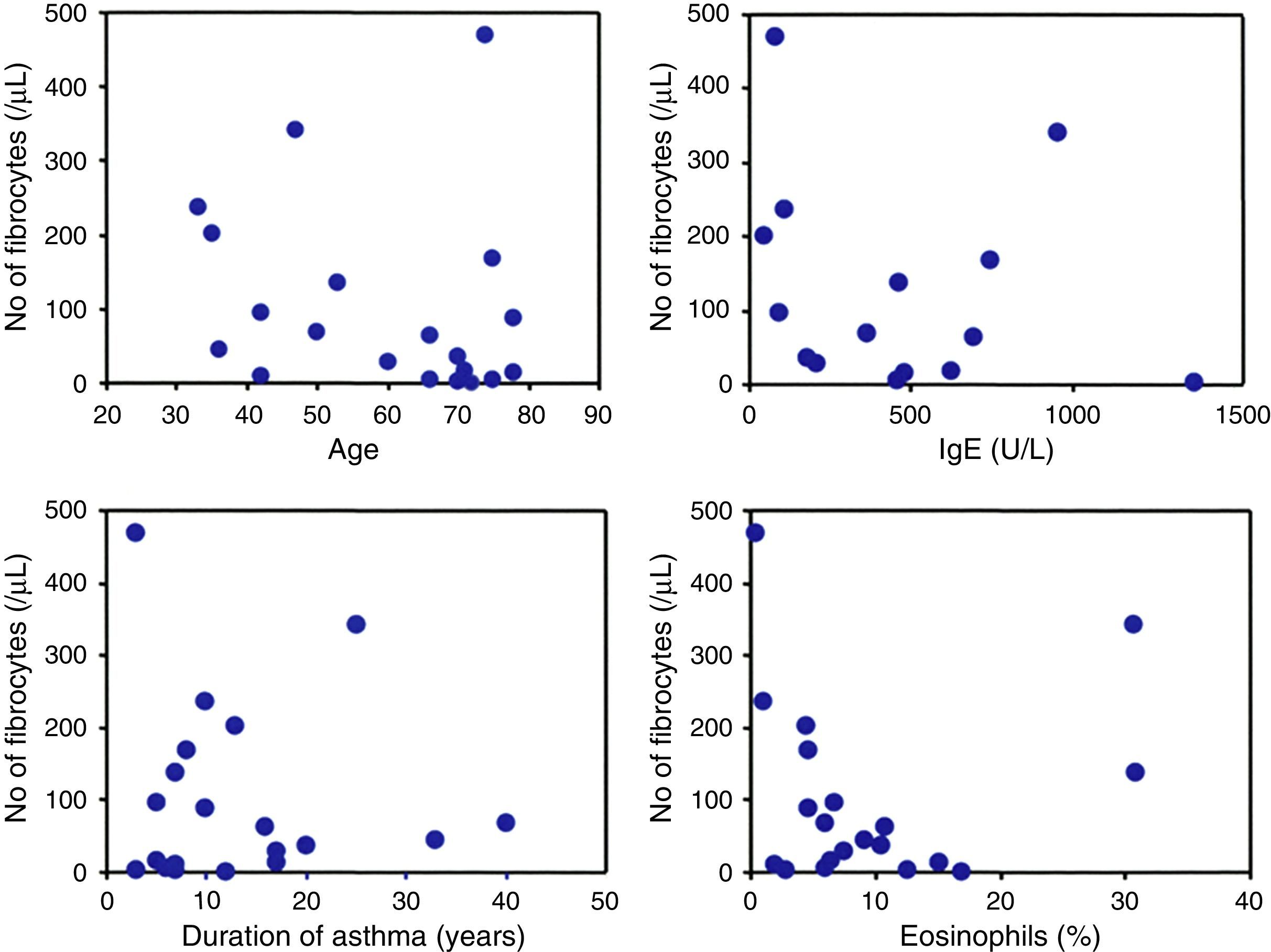
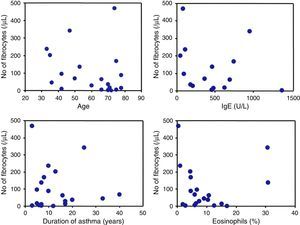
The number of circulating fibrocytes was significantly correlated with the frequency of rescue with inhaled SABA (r=0.9, p<0.05) and inversely correlated with the ACT score (r=−0.8, p<0.05) (Fig. 2B). The age, duration of asthma, percentage of peripheral blood eosinophils and serum IgE levels were not significantly correlated with the number of circulating fibrocytes (Fig. 3).
DiscussionAirway remodelling and fibrocytesA feature of bronchial asthma is airway remodelling.27 The general belief is that airway remodelling is a fibrotic response to long-lasting Th2-mediated inflammation but most recent evidence suggests that the onset of the structural remodelling of the airways may occur even at early stages of the disease.27 The mechanism is not completely clear but mechanical stress caused by bronchospasm, which is a characteristic early asthma sign, may by itself stimulate the secretion of pro-fibrotic cytokines such as transforming growth factor-β1 released from structural cells leading to enhanced secretion of ECM proteins from fibroblasts and myofibroblasts.28 Another contributing factor is the total population of myofibroblasts, and in this regard, the role of fibrocytes has been the focus of much attention because several studies have shown that they are the renewable source of myofibroblasts during the repair of tissue injury and fibrosis, and that they markedly accumulate into the airways with chronic inflammation, their recruitment being correlated with the severity of asthma.8–10,14 Consistent with these previous observations, the present study showed significantly enhanced number of circulating fibrocytes in patients with chronic asthma compared to a healthy control group, the number being proportional to the severity of the disease. Interestingly, the number of circulating fibrocytes was significantly correlated with parameters of airway limitation, suggesting that bronchoconstriction may also be involved in the increased homing of fibrocytes to the airways possibly by a chemokine-mediated mechanism as previously reported.17,29–32
Fibrocytes and composite score of asthma controlAn original and novel observation in the present study is the significant correlation of the number of circulating fibrocytes with ACT score and with the frequency of rescue by β2 agonist. ACT kit currently used as a tool for assessment of clinical control in asthma patients and consists of several questions on the frequency of symptoms, patients’ self-rating of control and the use of rescue bronchodilators.16,17,33 Previous studies have shown that ACT is a reliable parameter for assessing asthma control, prediction of exacerbation and for changing treatment strategies.16,19,33 There appears to be no doubt about the detrimental role of fibrocytes in the pathogenesis of airway remodelling.34 Thus, the fact that the number of circulating fibrocytes correlates with poor response to therapy (low ACT and frequent procaterol rescue) provides further evidence on the importance of asthma control for reducing the number of circulating fibrocytes as a measure to prevent airway remodelling.11 It is worth noting that circulating fibrocytes were not significantly associated with age, duration of asthma, serum IgE or with the percentage of eosinophils, suggesting that the number of circulating fibrocytes is determined by factors, probably chemokines, other than ageing, disease duration or allergic factors.34
In brief, the results of this study showed that the number of circulating fibrocytes is correlated inversely with ACT and proportionally with the frequency of SABA rescue in chronic bronchial asthma, further providing evidence that the number of circulating fibrocytes may be a marker of clinical control in asthma.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors declared no conflict of interest.
This study was supported by a grant-in-aids (No 26860605) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The funders had no role in study design, data analysis, decision to publish, or preparation of the manuscript.