Although the BCG vaccine remains the only available vaccine, a number of complications from local to systemic adverse reactions can occur.
ObjectiveThe aim of the study was to review the clinical features and treatment of Bacillus Calmette–Guérin (BCG) complications in children.
MethodsChildren with clinical and laboratory findings compatible with a diagnosis of local complication and disseminated disease at Masih Daneshvari Medical Center were enrolled from March 2013 to September 2015.
ResultsAmong 49 children with BCG complications, 35 (71%) had local complications and 14 (29%) had disseminated disease. The mean age at presentation was nine months (range: 1m–13y). The male to female ratio was 1.7:1. Suppurative lymphadenitis was seen in 25 of 35 (71%) cases. Among cases with disseminated disease, primary immunodeficiency (PID) was identified in nine (64%) cases. All cases with non-suppurative lymphadenitis were managed conservatively. Twenty (80%) cases with suppurative lymphadenitis were managed differently with medical treatment or surgery. In disseminated cases, three (43%) were treated with only medical treatment and eight (57%) with both medical and surgical treatment.
ConclusionsMost children with BCG complications had a local disease in our study. A higher rate of disseminated disease was also observed. In addition, PID was identified in most children with disseminated disease. Development of more appropriate BCG vaccines and changing the current vaccination programme in cases with suspected PID are required in our country.
The Bacillus Calmette–Guerin (BCG) vaccine, a live attenuated vaccine of Mycobacterium bovis, remains the only available vaccine against tuberculosis (TB) since 1921 despite attempts to produce novel generations.1 Despite the good protective efficacy of this vaccine, some complications with various degrees of severity can occur, varying from local to systemic adverse reactions.2 Local adverse reactions include erythema, induration, papule, injection site abscess, and lymphadenitis while systemic adverse reactions include osteomyelitis and disseminated BCG disease.3 Although the disseminated disease is rare, the case fatality rate of this disease is 80–85%.4 The rate of disseminated disease and lymphadenitis has recently increased in BCG vaccinated children.5,6 Moreover, a higher rate of disseminated disease is reported in Iran in comparison with previous rates.7 Although some studies have reported that the disseminated disease can occur in patients with a normal immune system, this disease is mainly associated with primary immune disorders (PID).8,9
We conducted this study to describe the complications of the BCG vaccine through assessing demographic data, clinical features, laboratory findings, and treatment in children admitted to a university hospital in Tehran, Iran.
MethodsThis study was conducted on all children who attended the paediatric clinic of National Research Institute of Tuberculosis and Lung Disease (NRITLD) in Masih Daneshvari Medical Center with any BCG complication between March 2013 and September 2015. In Iran, the BCG sub-strain Pasteur 1173 is administered to all new-borns.3
The complications associated with BCG vaccine were defined as the following conditions: (1) Local a: abscess at the injection site, isolated lymphadenitis, or both, b: lymphadenopathy >1cm with the presence of an abscess at the injection site over 12 weeks, (2) Systemic: disseminated BCG disease defined as a systemic syndrome consistent with mycobacterial infection such as weight loss, fever >38°C for more than two weeks, a family history of immunodeficiency, related parents, anaemia, hepatomegaly, and splenomegaly. Furthermore, mycobacterial infection was defined as the presence of acid fast bacilli and/or necrotising granuloma at two or more anatomic sites beyond the vaccination site as confirmed by histology. Immune functions were evaluated by the complete blood count, nitro blue tetrazolium test (NBT), flow cytometry, measurement of the level of immunoglobulins, and human immunodeficiency virus serology (HIV). Primary immunodeficiency disorders (PID) were diagnosed according to the criteria of the International Union of Immunological Societies (IUIS).10 Treatment was provided as conservative treatment (wait and watch), surgery including needle aspiration, total resection, and chest tube, and medical treatment (anti TB treatment: ATT). Statistical analysis was performed using SPSS version 16. Ethical approval was obtained from Shahid Beheshti University Paediatric Research Committee.
ResultsForty-nine children with BCG complication with a median age of 2.5 years (range: 4m–13y, mean: 3years) were evaluated during the 2.5 year period. The median duration between vaccination and complications was four months (range: 1m–13y, mean: 9 months). The male to female ratio was 1.7:1. Thirty-four (69%) children were younger than one year old. Twelve (25%) children were 1–2 years old, and three (6%) were older than two years of age. Thirty-five (71%) children had local complications and 14 (29%) had systemic complications.
Local complicationsDemographic dataAll of the 35 children who presented with local complications had received BCG vaccination at birth except for two children who received it at one month of age. Four (11%) children were preterm. The median birth weight was 3175g (range: 1960–3850, mean: 3.131g). Birth weight less than 2500g was found in one child (3%). Twenty-two (63%) children were male.
Clinical dataComplications were detected in children aged one month to two years (median: 11m). Twenty-eight (80%) children were younger than one year old. Thirty-four (97%) children had lymphadenitis and one (3%) child had abscess formation in the injection site. The sites of 34 children with lymphadenitis were reported as follows: axillary (n=21, 61%), cervical (n=4, 12%), axillary and supraclavicular (n=5, 15%), and axillary and cervical (n=4, 12%). Two (6%) lymphadenitis were ≤1cm, 16 (47%) were 1–3cm, and 16 (47%) were >3cm in size. Suppurative lymphadenitis was seen in 25 (71%) and non-suppurative lymphadenitis was seen in 10 (29%) patients. The duration of complications was reported as follow: <1month in two (6%), 1–3 months in three (8%), and >3 months in 30 (86%).
Laboratory dataThe result of TST was reordered for 13 children of whom four (30.7%) had TST >15mm. HIV was not detected in any children.
The abnormal finding of the blood profile included leucocytosis in five (14%). The erythrocyte sedimentation rate (ESR) was recorded in 20 children which was less than 10mm/h in 10 children and 10–50mm/h in 10 children.
Microbiological evaluations, including acid fast bacillus (AFB) smear, culture, and polymerase chain reaction (PCR), were performed in 20 children with normal results. Histologically, biopsies in six out of 20 (30%) children showed necrotising granulomas (with suppurative lymphadenitis in five and non-suppurative lymphadenitis in one).
No definite immunodeficiency was identified in 15 children evaluated for PID.
Radiographic dataChest radiography was available in 22 children who all had normal results.
Treatment dataAll children with non-suppurative lymphadenitis were conservatively managed. Of 25 children with suppurative lymphadenitis, seven (28%) received medical treatment and 13 (52%) were managed with incision drainage; the parents of five (20%) children did not consent to treatment.
Systemic complicationsDemographic dataAll of the 14 children that were identified with disseminated BCG disease had received BCG vaccination at birth. Nine (64%) children were male. All children were full-term. The median birth weight was 3300g (range: 2730–4390, mean: 3448g). Complications were noted between 1 and 18 months of age (median: 3m). Twelve (86%) children were less than six months.
Clinical dataA family history of TB, death, and immunodeficiency was found in one (7%), three (21%), and three (21%) children, respectively. The parents of 11 (79%) children were relatives. The primary clinical presentation of children at the onset of the disease was left axillary lymphadenopathy (LAP) in nine (64%), right axillary LAP in two (14%), fever in one (7%), pleural effusion (PE) in one (7%), and multiple LAP in one (7%). The common features were LAP in nine (64%), fever in seven (50%), cough in four (28.5%), weight loss in two (14%), and loss of appetite in one (7%).
Laboratory dataThe result of TST was reordered for nine children of whom three (33%) had TST >15mm. HIV was not detected in any children.
The abnormal findings of the blood profile included anaemia in 11 (78.5%), thrombocytopenia in one (7%), leukocytosis in two (14%), and thrombocytosis in six (42%). The erythrocyte sedimentation rate (ESR) was recorded in 14 children; the result was less than 10mm/h in two children, 10–50mm/h in five children, and more than 50mm/h in seven children. NBT was performed in 11 children who all (100%) had NL results. C-reactive protein (CRP) was measured in 12 children of whom eight (67%) had abnormal results.
A positive AFB smear was identified in seven out of 13 (54%) children; the specimens were obtained from gastric washing (GW) in four, lymph node (LN) in two, and bone morrow (BM) in one. A positive culture for Mycobacterium tuberculosis (M.TB) was found in five out of 10 (50%) children; the specimens were obtained from GW in three, LN in one, and BM in one patient. PCR was performed in 10 children of whom seven (70%) children had positive results; the specimens were provided from GW in three, LN in two, and BM in two patients. The result of PCR for M. bovis was positive in two children and the result of PCR for M.TB complex was positive in five children. Histologically, biopsies in seven (50%) children showed necrotising granulomas; the sites included the LN in three, BM in two, bronchus and liver in one, and LN and liver in one.
Definite immunodeficiency was identified in nine (64%) children: SCID in two (14%), CGD in two (14%), interleukin-12 (IL 12) deficiency in two (14%), MSMD in two (14%), and MSMD and IL12 deficiency in one (7%).
Radiographic dataChest radiography was performed in 10 children of whom four (40%) had abnormal findings including parenchymal involvement in two children, LAP in one child, and PE and pericardial effusion in one child. Abdominal sonography was performed in all children of whom 10 (71%) had abnormal results: LAP in two children, hepatosplenomegaly in two children, LAP and hepatosplenomegaly in five children, and PE and prenephric abscess in one child.
Treatment dataOf 14 children, six (43%) received medical treatment alone and eight (57%) underwent both medical and surgical treatment. As for medical treatment, two children received 2-drug ATT (INH and RIF), eight children received 4-drug ATT and INF, and four children received ≥4-drug ATT without INF. Of the eight children who received surgical treatment, five were managed by incision and drainage, two underwent total resection of LN, and one had chest tube for PE. Comparison of demographic and clinical variables between patients with local and systemic BCG complications is presented in Table 1.
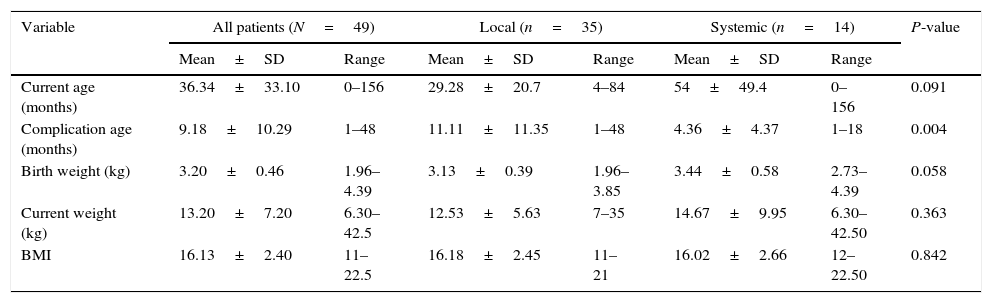
Comparison of demographic and clinical variables between patients with local and systemic BCG complications using t-test.
| Variable | All patients (N=49) | Local (n=35) | Systemic (n=14) | P-value | |||
|---|---|---|---|---|---|---|---|
| Mean±SD | Range | Mean±SD | Range | Mean±SD | Range | ||
| Current age (months) | 36.34±33.10 | 0–156 | 29.28±20.7 | 4–84 | 54±49.4 | 0–156 | 0.091 |
| Complication age (months) | 9.18±10.29 | 1–48 | 11.11±11.35 | 1–48 | 4.36±4.37 | 1–18 | 0.004 |
| Birth weight (kg) | 3.20±0.46 | 1.96–4.39 | 3.13±0.39 | 1.96–3.85 | 3.44±0.58 | 2.73–4.39 | 0.058 |
| Current weight (kg) | 13.20±7.20 | 6.30–42.5 | 12.53±5.63 | 7–35 | 14.67±9.95 | 6.30–42.50 | 0.363 |
| BMI | 16.13±2.40 | 11–22.5 | 16.18±2.45 | 11–21 | 16.02±2.66 | 12–22.50 | 0.842 |
In this study conducted on children with BCG vaccine complications during a 2.5 year period, approximately two-thirds of the study population had local complications and one-third had disseminated BCG disease. As for local complications, most cases presented with suppurative lymphadenitis. Definite immunodeficiency disorder was recognised in 64% of the cases with disseminated disease. All cases with non-suppurative lymphadenitis were managed conservatively. The majority of the cases with suppurative lymphadenitis were differently managed with medical or surgical treatment. Also, cases with disseminated disease were treated with only ATT or with both ATT and surgery.
Local complications occur in about 1/1000 children who receive BCG vaccine.11,12 Possible factors such as impaired Th1 immunity in early life, BCG overdose, vaccine strain, interadermal technique, underlying immunodeficiency, or HIV can cause complications. In addition, younger children are more vulnerable to complications.13,14 In our study, the most common complication of the BCG vaccine was the local complication of suppurative lymphadenitis. This finding was similar to that of other studies.2,9,15,16 We found injection site abscess in only one child that was consistent with a study conducted by Mahmoudi et al.15
About 1.4 million infants receive BCG vaccine every year in Iran.7 Few population-based studies are available on the prevalence of BCG complications in Iran. According to one recent study in Iran, among 15,984 BCG vaccinated infants, local complications occurred in 150 (0.93%) cases and disseminated disease was detected in three (0.02%).17 However, the number of the cases of disseminated BCG disease is estimated to be higher than the previous rate in Iran.7 Possible factors include the higher reactogenicity of Pasteur 1173 strain and the higher prevalence of PID in Iranian children. New strategies may be required to approach the Pasteur strain in our country. For example, In Taiwan, the Pasteur strain has been replaced with the Tokyo strain which is less reactogenic.18 In addition, the Pasteur strain has been removed from the vaccination schedule in Austria since 1990.19
Overall, a disseminated disease occurs in 2–3.4permillion children who receive BCG vaccine.4 Systemic complications often occur within six months after BCG vaccination.4 In our study, among patients with disseminated BCG, the majority of the children were below six months of age. PID (chronic granulomatous disease, severe combined immunodeficiency, Mendelian Susceptibility to Mycobacterial Disease (MSMD), or disorders of the gamma-IFN/IL-12 pathway) are commonly found in children with disseminated disease.4 We found that the immunodeficiency rate was 64%, and the majority of our cases had IL-12 deficiency and MSMD. One of the main immunodeficiency diseases that can lead to disseminated disease is the disorder of the IL-12/IFN-γ axis that is now known as MSMD.6 HIV-infected children are at a great risk of disseminated BCG disease.20 No HIV positive cases were detected in our study, which was similar to studies by Paiman et al. and Indumathi et al.6,21
We found no immunodeficiency disorder in 36% of cases with disseminated disease. Similar findings have been noted in other studies.22,23 This finding indicates that the BCG vaccine has potential risks for all infants even if the infants have no immunodeficiency disorder.
We found that 70% of the cases with disseminated disease had related parents, which was similar to other studies conducted in Iran.5,23,24 Also, 21% of our cases had a history of immunodeficiency in their family. Regarding the high rate of cognation in Iranian families and the fact that most cases with disseminated BCG disease had PID, BCG vaccine should be administered after a complete immunological work-up has ruled out underlying immunodeficiency.15
The treatment of local complications of BCG with ATT is challenging due to inherent resistance of M. bovis to pyrazinamide and lack of drug penetration into the abscess cavity.11,14,19 Furthermore, the routine use of ATT cannot decrease the possibility of the conversion of non-suppurative lymphadenitis into the suppurative form.11,24 Surgical excision is the definite method to promptly cure suppurative lymphadenitis that causes a suitable wound recovery. Nevertheless, surgical manipulation and general anaesthesia are risky for patients, especially infants.4 Consequently, surgical excision must be considered in cases with a lymph node diameter more than 3cm, in cases with failed needle aspiration, and when the lymph nodes are matted and multioculated.25 Incision and drainage is not suggested due to delayed wound healing, unsatisfactory scarring, and persistent wound discharge.26,27 Unfortunately, incision and drainage was performed in 52% and medical treatment was started in 28% of our cases with suppurative lymphadenitis. According to recent studies, needle aspiration can decrease the risk of sinus formation in suppurative lymphadenitis and increase the regression of lymphadenitis.11,28 The new strategy for the management of suppurative lymphadenitis with needle aspiration and excision surgery should be employed in our setting.
In disseminated BCG disease, consultation with paediatric infectious diseases specialists and paediatric immunologists should be considered to administer ATT and identify PID, respectively. In addition, optimising the treatment, for example IFN-γ treatment in patients with CGD and haematopoietic stem cell transplantation (HSCT) in patients with SCID after identification of PID, is helpful.29,30 Several attempts are needed to reduce the complications of BCG vaccine, including standardising the method and dosage, screening for cases with a family history suggestive of PID prior to vaccination, especially with the expected existence of autosomal recessive forms, and safe vaccine production.
This study evaluated all children with BCG complications identified over a 2.5-year period in one university hospital. However, our study has some limitations. Since this study was a hospital-based study, and because a large number of children with local complications are managed as outpatients, we were unable to estimate the complication rate of the BCG vaccine.
Also, the complications of the BCG vaccine were not strictly followed-up. Moreover, the short follow-up duration was not appropriate for the evaluation of treatment response and mortality.
In conclusion, we found that most children with BCG complications had local complications. A higher rate of disseminated disease was also observed. Although most children with disseminated disease in our study were immunocompromised, we found no immunodeficiency disorder in some children. Development of more appropriate BCG vaccines and changing the current vaccination programme in the cases with suspected PID are required in our country.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Financial disclosureThere are no financial relationships relevant to this article to disclose.
Conflict of interestThere is no conflict of interest in this study.
This study was funded by National Research Institute of Tuberculosis and Lung Disease (NRITLD).