Asthma is a complex genetic disorder. Several genes have been found associated with asthma. The cystic fibrosis transmembrane conductance regulator (CFTR) gene is one of them. The aim of this study was to perform a comparative analysis of the genotype and allele frequency distributions of the biallelic marker M470V within the CFTR gene on mutant and wide chromosomes.
Patients and methodsThe molecular approach consists in the genotyping of the M470V marker by the PCR-RFLP technique in 105 asthmatic patients, aged between four months and 17 years, and 105 healthy subjects.
ResultsWe found a significant difference in the genotype frequencies between the two studied groups (χ2=9.855, P=0.007). The V/V genotype was over represented in the asthmatic group as compared to the controls (32.38% vs. 16.19%). Whereas, the M/V genotype is more frequent in healthy subjects (40.95% vs. 28.71%). We also noted a significant difference in allelic distribution of M470V with associated diseases (χ2=9.610, P=0.022).
ConclusionsThe present study is the first report on the distribution of the M470V polymorphism in asthmatic Tunisian patients. We noticed that the M470V variant could modulate the clinical phenotype of asthmatic patients. This preliminary study will establish the molecular basis of this disease in Tunisia.
Asthma is a common chronic disease of children and adults, which is characterized by airway inflammation, hyperactivity, mucus overproduction and airway obstruction.1 The airway epithelial cell is a prominent contributor to asthmatic exacerbations. Epithelial cell mucus production is increased in asthma,2 and plugging of the airways with mucus is sometimes fatal for severe asthma exacerbations.3 In Tunisia, the prevalence of current asthma was 6.5%.4 Asthma prevalence has increased worldwide over the last decades, and there is increasing evidence of gene–environment interactions in disease development.5 The Genetic Association Database (http://geneticassociationdb.nih.gov) has registered over 500 genetic association studies to asthma.6 Among the candidate genes is the cystic fibrosis transmembrane conductance regulator (CFTR) gene on chromosome 7q32.7
The CFTR gene codes for the cystic fibrosis (CF) transmembrane conductance regulator protein. CFTR transports both chloride and bicarbonate ions across the epithelial surface of many tissues; in the airway, this provides the epithelial surface with adequate hydration, osmotic pressure and pH regulation. Deficient CFTR anion transport and thereby reduced chloride and bicarbonate concentrations at the airway surface, leads to decreased mucus clearance, copious airway obstruction and loss of lung function.8 Changes in ion transport have also been implicated in asthma pathogenesis.9 More than 2000 CFTR mutations are described conferring different degrees of CFTR protein malfunctions (http://www.genet.sickkids.on.ca/cftr/). Some studies demonstrate positive association of the CFTR gene with asthma while others show either protective or no association.10 A common polymorphism in European populations, M470V, was the subject of several works in CF and CF-related diseases.10–12 In Tunisia, the M470V marker has been studied in congenital bilateral absence of the vas deferens (CBAVD) group13 and cystic fibrosis patients.14
To further investigate the contribution of polymorphisms in the CFTR gene in the asthmatic Tunisian population, the present work aims at performing a comparative analysis of the genotype and allele frequency distributions of the biallelic marker M470V located in exon 10 of the CFTR gene on mutant and wide chromosomes.
Materials and methodsSubjectsA total of 105 unrelated children, presenting a clinical course of asthma, were investigated. Patients (4 months to 17 years old) were collected by pediatric departments in the children's hospital Bechir Hamza of Tunis. The median age of diagnosis is 72 months.
An information sheet has been established for each patient analyzed containing: patient identification, characteristics of asthma (screening age, crisis number, hospitalization), socio-demographic data (origin, address, tobacco and pollution), last prescribed drugs for asthma, family study (antecedent of the disease and consanguinity), coexistence of atopic manifestation (rhinitis, dermatitis and conjunctivitis) and biological examination (hemoglobin level and skin test).
Asthma diagnosis was made in accordance with the American Thoracic Society (ATS).15 In short, current asthma was defined as recurrent wheezing or coughing in the absence of a cold in the preceding 12 months with a physician's diagnosis, and bronchial hyperresponsiveness upon methacholine challenge (PC20 ≤ 16mg/ml) or at least 12% reversibility of forced expiratory volume in 1 (FEV1) after inhalation of β2 agonist.16 The census information on asthma patients was carried out by an interview with one or both parents. In parallel, a control population of 105 healthy subjects was included in our study. Healthy children were age-matched to asthma subjects. They had no history of wheezing, recurrent or chronic diseases and infection during the preceding two weeks. All subjects did not have any other disease history, including pancreatic diseases. Written consent was obtained from all participants before enrollment in the study.
Genotyping of M470VWhole blood was obtained from each subject and genomic DNA was extracted by using a “Salting out” protocol.17
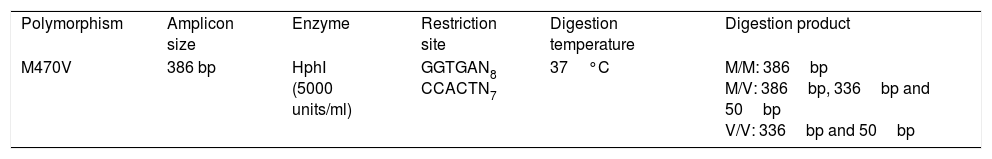
The M470V genotyping was analyzed by a restriction fragment length polymorphism (RFLP). The exon 10 containing the single nucleotide polymorphism (SNP) was amplified by PCR reaction which was carried out using the forward primer 5′TGATAATTGACCTAATAATGAT-3′ and the reverse primer 5′CATTCACAGTAGCTTACCCA-3′ resulting in an amplified fragment of 386bp in size. The PCR reaction mixture contained one unit of Thermus aquaticus DNA polymerase (Taq DNA Polymerase Recombinant, Invitrogen, USA, Error rate of approximately 1×10−5 errors per base), 1X Buffer, 0.2mM dNTP, 0.04pmol/μl of etch primers and 100ng DNA template. Cycling for reaction was performed as follows: 94°C for 5min for initial denaturation, 30 cycles at 94°C for 30s, 54°C for 30s, and 72°C for 30s, followed by one cycle at 72°C for 5min for final extension. To make sure of the good conditions of the PCR reaction, positive (a nucleic acid solution) and negative (Mix not containing the target nucleic acid replaced by ultrapure water) controls were used. The PCR product is then digested with 5U of the HphI restriction enzyme (BioLabs, New England, USA), in a mix containing 2.5μl of buffer (10X NEBuffer) and 0.5μg of DNA and ultrapure water, at 37°C temperature for 4h (Table 1). The M470V polymorphism creates the restriction site of the HphI enzyme.
Samples of PCR products and digested fragments were checked by electrophoresis on 1.5% and 4% agarose gel respectively with Red Safe in the presence of a molecular size marker (100bp-Ladder Promega).
To verify the correct process of the PCR-RFLP, two positive controls are used: the first is a heterozygous control (M/V) and the second is a positive control (V/V). The genotype of these controls was obtained via sequencing DNA.
Statistical analysisStatistical data analysis was performed on Statistical Package for the Social Science (SPSS) versions 20.0 for windows. Chi2 test was used to compare genotypes and allele distribution. Differences were considered statistically significant as P<5%. Hardy–Weinberg equilibrium (HWE) was tested for M470V locus among asthma and control cohorts.
ResultsRetrospective study of asthma casesThe clinical characteristics of the 105 asthmatic patients were analyzed for phenotypic and epidemiological data. The majority of Tunisian regions are represented in our sick population; 54.1% of the patients are from the Northwest and 18.4% from the Northeast. The sex ratio is 1.85 in favor of boys. A percentage of 29.5% of patients are from consanguineous couples. This relatively high rate of consanguinity is characteristic of the Tunisian population.18 Half of the patients (53.3%) have a family history of asthma. The skin test performed for all patients revealed that 60.78% had an allergy of which 67.74% was due to dust and pollen. The search for associated diseases showed that 55.17% of patients present allergic rhinitis, 20.68% conjunctivitis, 13.79% atopic dermatitis and 12% suffer from anemia. We also noted that 61.9% of our patients are passive smokers who inhaled smoke that comes from someone else smoking (mostly from parents).
Genotype frequencies in asthma and non-asthma groupsThe common CFTR amino acid variant M470V is a missense mutation located in the exon 10 of the CFTR gene and leading to a substitution of an adenine (A) by a guanine (G) (A>G) at position 1540 of the Nucleotide sequence, which results in a replacement of the amino acid methionine (Met) with a Valine (Val) at position 470 of the peptide sequence.
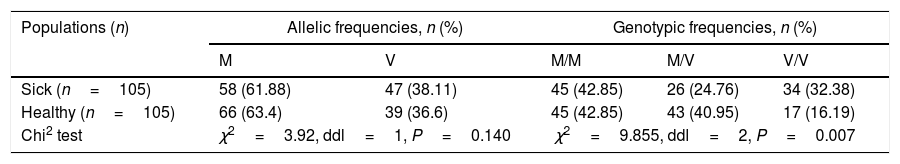
To investigate the association between M470V and asthma, a case-control study was performed for 105 patients. The distribution of M470 and V470 alleles among normal and patient groups was consistent with the Hardy–Weinberg equilibrium (HWE) (P=0.5557 in controls and P=0.55 in asthmatic patients). The genotypic and allelic distribution of M470V into the non-asthma and asthma groups is summarized in Table 2. No significant differences in allelic distribution were observed between the two groups (χ2=3.92, P=0.140). The M allele frequency was higher compared with V allele in both groups (61.88% vs. 38.11% in asthma group and 63.4% vs. 36.6% in control group).
Genotypic and allelic distributions of the M470V polymorphic site in mutant and normal chromosomes.
| Populations (n) | Allelic frequencies, n (%) | Genotypic frequencies, n (%) | |||
|---|---|---|---|---|---|
| M | V | M/M | M/V | V/V | |
| Sick (n=105) | 58 (61.88) | 47 (38.11) | 45 (42.85) | 26 (24.76) | 34 (32.38) |
| Healthy (n=105) | 66 (63.4) | 39 (36.6) | 45 (42.85) | 43 (40.95) | 17 (16.19) |
| Chi2 test | χ2=3.92, ddl=1, P=0.140 | χ2=9.855, ddl=2, P=0.007 | |||
P<0.05 is considered statistical significance.
n: number.
Concerning the genotype distribution of M470V, there was a significant difference in the genotype frequencies between the two studied groups (χ2=9.855, P=0.007). The V/V genotype was over represented in the asthmatic group as compared to the controls (32.38% vs. 16.19%). Whereas, the M/V genotype is more frequent in healthy subject (40.95% vs. 28.71%).
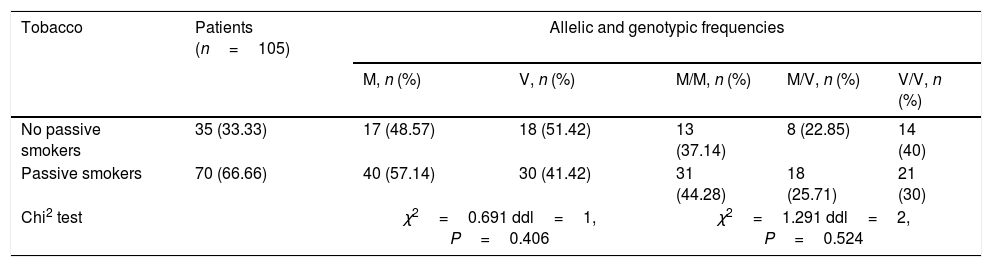
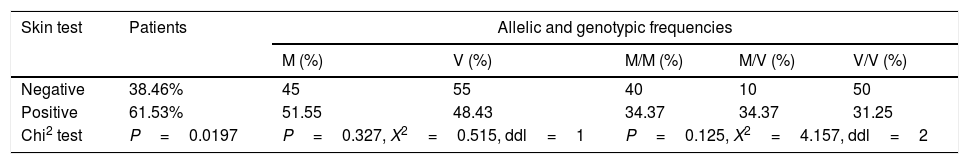
In order to show a possible association between M470V polymorphism and tobacco, skin test, allergic rhinitis, conjunctivitis, dermatitis and anemia a statistical study was carried out (Tables 3–5). No significant difference was noted between the no passive and passive smokers in allelic and genotypic distribution (Table 3). Moreover, we did not notice any significant difference with the skin test (Table 4).
Allelic and genotypic distribution of M470V with no passive and passive smokers.
| Tobacco | Patients (n=105) | Allelic and genotypic frequencies | ||||
|---|---|---|---|---|---|---|
| M, n (%) | V, n (%) | M/M, n (%) | M/V, n (%) | V/V, n (%) | ||
| No passive smokers | 35 (33.33) | 17 (48.57) | 18 (51.42) | 13 (37.14) | 8 (22.85) | 14 (40) |
| Passive smokers | 70 (66.66) | 40 (57.14) | 30 (41.42) | 31 (44.28) | 18 (25.71) | 21 (30) |
| Chi2 test | χ2=0.691 ddl=1, P=0.406 | χ2=1.291 ddl=2, P=0.524 | ||||
P<0.05 is considered statistical significance.
n: number.
Allelic and genotype distribution of M470V with skin test.
| Skin test | Patients | Allelic and genotypic frequencies | ||||
|---|---|---|---|---|---|---|
| M (%) | V (%) | M/M (%) | M/V (%) | V/V (%) | ||
| Negative | 38.46% | 45 | 55 | 40 | 10 | 50 |
| Positive | 61.53% | 51.55 | 48.43 | 34.37 | 34.37 | 31.25 |
| Chi2 test | P=0.0197 | P=0.327, X2=0.515, ddl=1 | P=0.125, X2=4.157, ddl=2 | |||
P<0.05 is considered statistical significance.
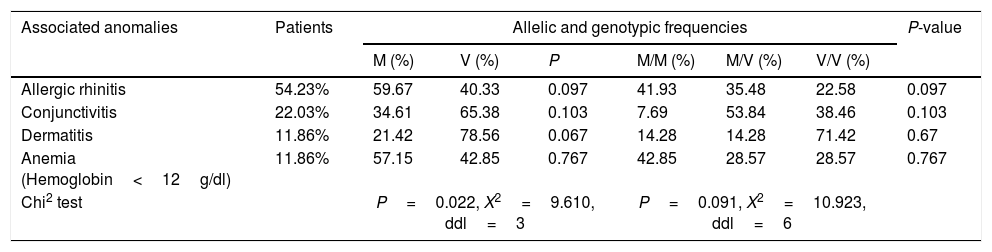
Allelic and genotypic distribution of M470V with associated diseases.
| Associated anomalies | Patients | Allelic and genotypic frequencies | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| M (%) | V (%) | P | M/M (%) | M/V (%) | V/V (%) | |||
| Allergic rhinitis | 54.23% | 59.67 | 40.33 | 0.097 | 41.93 | 35.48 | 22.58 | 0.097 |
| Conjunctivitis | 22.03% | 34.61 | 65.38 | 0.103 | 7.69 | 53.84 | 38.46 | 0.103 |
| Dermatitis | 11.86% | 21.42 | 78.56 | 0.067 | 14.28 | 14.28 | 71.42 | 0.67 |
| Anemia (Hemoglobin<12g/dl) | 11.86% | 57.15 | 42.85 | 0.767 | 42.85 | 28.57 | 28.57 | 0.767 |
| Chi2 test | P=0.022, X2=9.610, ddl=3 | P=0.091, X2=10.923, ddl=6 | ||||||
P<0.05 is considered statistical significance.
However, a significant difference was found in allelic distribution of M470V with associated disease (χ2=9.610, P=0.022). In fact, we observed that the V allele is more common than the M allele in patients with conjunctivitis or dermatitis with 65.38% versus 34.61% in conjunctivitis and 78.56% versus 21.42% in dermatitis (Table 5). However, the M allele is the most dominant in patients with allergic rhinitis or anemia with 59.67% and 57.15% respectively.
DiscussionM470V polymorphism is a missense mutation located in exon 10 coding for the first nucleotide-binding domain of the CFTR protein; it reaches high frequencies in the general population.17 This marker has been the subject of numerous works in the world. It has been studied primarily in patients with cystic fibrosis,11,14 bilateral agenesis of the vas deferens (ABCD),19 idiopathic chronic pancreatitis (ICP)20 and also in the general population (healthy subjects).21
Few studies have been conducted in asthmatic patients about genotypes and allelic distribution of M470V and its implication on the clinical variability and severity of the disease. The present work is the first Tunisian study to investigate the association between M470V polymorphism and asthma. The allelic distribution showed no significant difference between the two study populations where the M allele is the more frequent in asthmatic and normal subjects with 61.84% and 63.33%, respectively. However, a significant difference in genotype distribution of M470V was found between the two studied populations. This result is similar to a recent study conducted in Tunisian cystic fibrosis patients where the M/M genotype is the most frequent with 82%.14 However, our results are contradictory with studies from the French,11 Chinese,23 Korean24 and Spain populations25 where M/V genotype and V allele are more represented. Furthermore, Hakonarson et al. failed to show evidence of a linkage between asthma and chromosome 7q31.2.26 These inconsistent results are probably due to different ethnic origins. In fact, the geographical distribution of the frequency of M470V polymorphism observed in a healthy population shows that the V470 allele is more common than the M470 allele in European and Asian populations.27,28 In contrast, the M470 is more frequent in the African population.
Interestingly, functional studies of the M470V variant have identified a two-fold increased chloride channel activity for M470 with respect to V470.29 Therefore, Tzetis et al. demonstrate that M470V affects the biogenesis of CFTR protein and the gating of the CFTR channel.30 It has been postulated that abnormalities in airway chloride transport may contribute to the pathogenesis of asthma. It has also been suggested that CFTR protein can also regulate other chloride channels.31 Thus, it cannot be excluded that a putative hyperfunction of CFTR due to missense mutations, perhaps influenced by the presence of the M470 allele, could have functional consequences in several cell types of the airway.
M470V has long been considered a non-pathogenic polymorphism. However, M470V is not strongly neutral because Ciminelli et al. showed that this polymorphism leads to degradation of chloride transport through the cell's membrane. It has also been noted that whenever M470V polymorphism comes along with another polymorphism or mutation leads to less activation of CFTR protein function.22 It should be noted that M470V behaves like a factor of genetic predisposition for the development of certain CFTR-related diseases.32
It is known that the CFTR is a chloride channel located in the apical membrane of epithelial cells in airways, intestines, ducts of the pancreas, and sweat glands. However, CFTR expression is not limited to epithelial cells. Some studies show that the CFTR channel is present in airway smooth muscle cells, and that it modulates the release of Ca(2+) in response to contractile agents. In CF patients, a dysfunctional of CFTR channel could contribute to the asthma diathesis.33,34
A statistical study was also conducted in order to show a possible association between M470V polymorphism and tobacco, skin test and associated diseases. Our result does not appear to modulate tobacco and skin test. Indeed, in our cohort, 61.53% had a positive skin test; this result is supported by R de Cid et al. who found a positive association between the skin test and asthma.11 On the other hand, Lázaro et al. noted that the prevalence of positive skin test reactivity was lower in the group with CFTR missense mutations, although this difference was not statistically significant.26 Concerning the effect of passive smoking on asthma, our study showed no positive association, contrary to studies in the literature suggesting that passive smoking exacerbates asthma.35 There is already an extensive literature; reviewed elsewhere, showing that passive smoking is associated with an increased risk of asthma in children and adults.36 Other works demonstrated that passive smoke exposure exacerbates symptoms in asthmatic patients. These latter findings are supported by the results of experimental challenge studies indicating that passive smoke exposure has adverse effects on airflow and/or airway responsiveness in asthma.37 This lack of a positive association between passive smoking and asthma in our studied population can be explained by the fact that our data on passive smoking were limited. In fact, secondhand smoke exposure is difficult to ascertain and quantify. In fact, the best estimate of the association between lifetime passive smoke exposure and children asthma requires good measures of passive smoke exposure (duration and frequency of exposure, age of the children at the first exposure).
However, a significant difference was observed between the genotype distribution of M470V and associated diseases (P=0.022). This finding suggests that this variant may be involved in the variability of clinical expression of asthma.
A percentage of 54.23% of our patients have allergic rhinitis (AR), suggesting its association with asthma. A recent Tunisian study also showed that almost half of asthmatics have rhinitis or rhinoconjunctivitis.4 These results are supported by Kim et al. who found that 30–80% of asthmatic patients presented AR.38 Simons suggested that these findings were probably underestimates of the relationship, because more recent, sensitive interview protocols found rhinitis in 98.9% of allergic subjects with asthma and in 78.4% of non-allergic subjects with asthma.39 Leynaert et al. reviewed several studies demonstrating the strong association between rhinitis and asthma, both allergic and non-allergic subjects.40 Other studies showed that dermatitis has been associated with an increased risk of asthma.41
Gradman and Wolthers described that 24% of children with asthma had concomitant allergic conjunctivitis with rhinitis, which is in concordance with our finding. The allergens may be suggested to be triggers of common pathophysiologic inflammatory mechanisms in the conjunctiva and in the nasal and respiratory mucosa.42
ConclusionAsthma represents a considerable public health problem in children. The present study is the first report on the distribution of the M470V polymorphism in Tunisian asthmatic patients. We first showed that the V/V genotype is more frequent in asthmatic patients compared to healthy subjects. Second, we noticed that the M470V variant could modulate the clinical phenotype of asthmatic patients. Further studies at several levels are needed to define the potential contribution of M470V with other CFTR mutation to the pathogenesis of asthma.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
FundingThis work was supported by a Grant from the Research Laboratory (LR00SP03) “Ministry of Higher Education and Scientific Research” of Tunisia.
Conflict of interestThe authors have no conflict of interest to declare.