The comparison of smokeless tobacco (ST) exposure versus Ovalbumin (Ova) sensitized rats or asthmatic patients has hardly been studied in the literature. Thus, the present study aims to investigate the aggravation of inflammation, exacerbation of asthma, oxidative stress and cytotoxicity induced by ST.
MethodsST was given at the dose of 40mg/kg in an allergic asthma model in Wistar rats. Furthermore, the effects of oral administration of Nigella sativa oil (NSO), at a dose of 4mL/kg/day, were investigated.
ResultsThe obtained results showed that ST clearly enhanced lung inflammation through interleukin-4 (IL-4) and Nitric oxide (NO) increased production. Actually, ST was found to intensify the oxidative stress state induced by Ova-challenge in rats, which was proven not only by augmenting lipid peroxidation and protein oxidation, but also by altering the non-enzymatic and enzymatic antioxidant status. Furthermore, the aggravation of inflammation and oxidative stress was obviously demonstrated by the histopathological changes observed in lung. In contrast, NSO administration has shown anti-inflammatory effects by reducing IL-4 and NO production, restoring the antioxidant status, reducing lipid peroxidation and improving the histopathological alterations by both protein oxidation and NSO treatment.
ConclusionsOur data have proven that severe concurrent exposure to allergen and ST increases airway inflammation and oxidative stress in previously sensitized rats. They also suggest that the oral NSO treatment could be a promising treatment for asthma.
Over the past two decades, toxicological research has been interested in the induction of oxidative stress after the consumption of smokeless tobacco (ST) as a possible mechanism of toxicity in liver, kidney and lung.1–3 Presumably, the direct contact of the respiratory epithelium with ST toxic compounds, even when it is not burned or volatilized, can cause inflammatory changes.1
Allergic asthma affects about 300 million people of all ages worldwide. It is increasing by 50% per decade4 and is considered a very serious public health problem. Allergic inflammation is also frequently associated with an increased generation of reactive oxygen species (ROS),5 and the biochemical environment in the asthmatic airways is favorable for free radical mediated reactions. It has been shown that inflammation caused by increased oxidative stress occurs in the airways of patients with asthma.5
Moreover, it would be interesting to study the ST additive effects on an experimental asthma model, while seeking a natural way to remedy the two sources generating inflammation and oxidative stress. Therefore, we investigated the traditional remedies of pulmonary pathologies and chose Nigella sativa, since it is known in the prophetic writings that it heals all the evils.6 This plant, commonly known as black seed, is an annual herbaceous plant belonging to the Ranunculaceae family.
As previously mentioned, N. sativa is known for its pharmacological and therapeutic effects, namely due to its antibacterial, antifungal, antidiabetic, antioxidant, anti-inflammatory, anticancer and immunomodulatory properties.7,8 Many active compounds have been isolated and identified through phytochemical investigations such as phenolic acid, epicatechin, quercetin and flavones.9 Moreover, most of the therapeutic properties of this plant are related to the presence of thymoquinone (TQ) which is the main active chemical component cold-pressed black cumin seed oils10 when linoleic, oleic and palmitic acids were the main fatty acids in the oils tested. Otherwise, in Ramadan study,11 the author showed that cold-pressed oils of N. sativa were healthy oils and had good anti-free radical properties and good oxidation stability, probably due to changes in the fatty acid profile and tocols.11
The present study explores not only the effects of ST in the aggravation of inflammation, but also the preventive and ameliorating effects of N. sativa oil (NSO) on allergen-induced airway inflammation in a rat model of allergic asthma associated with taking ST.
Materials and methodsAnimalsForty-eight Wistar albino male rats weighing 160±10g (6–8 weeks old), obtained from the Pasteur Institute (Algiers, Algeria) were used. The animals were housed in polypropylene cages that were sanitized every 48h. The rats were fed a standard laboratory diet (standard food, supplied by the “ONAB of Bejaia”, Algeria) and clean tap water ad libitum. They were exposed to a natural photoperiod, at a temperature of 25±1°C and a relative humidity of 40±5% and allowed to acclimatize in this condition for two weeks prior to experimental use. All protocols in this study were used in accordance with the guidelines of the Committee on Use of Laboratory Animals and approved under the CNEPRU project (D01N01UN230120150006) by the Ethical Committee of DGRSDT at the Algerian Ministry of Higher Education and Scientific Research.
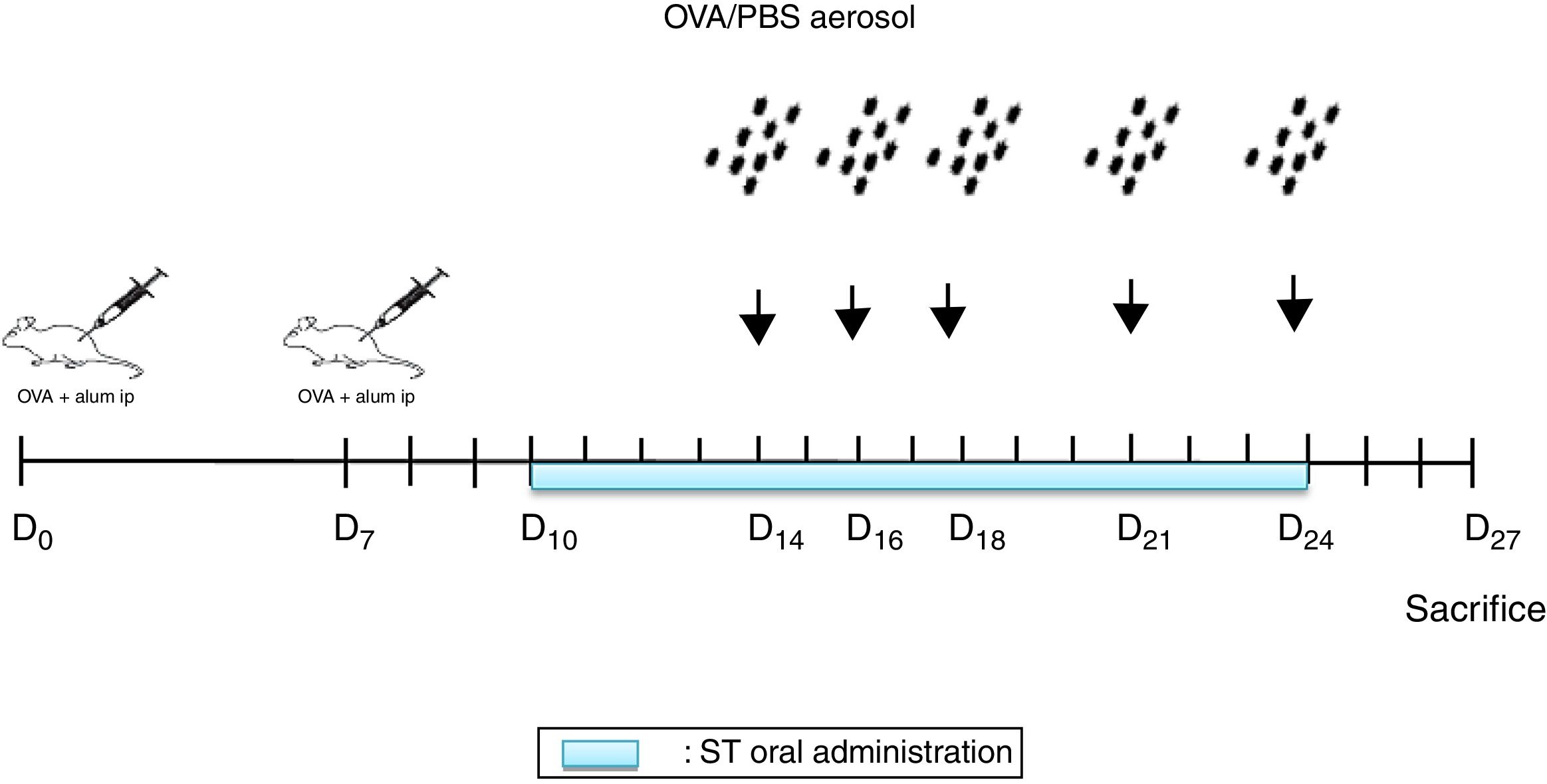
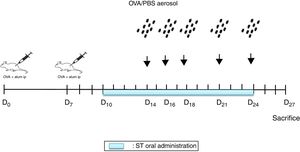
Sensitization and aerosol exposureThe rats were immunized by an intraperitoneal injection of 10mg Ovalbumin (Ova) adsorbed to 1mg aluminum hydroxide in a volume of 1mL phosphate buffered saline (PBS) on day 0 and boosted on day 78,12 (Fig. 1).
NSO was administered 4 days before the first injection. 2 Ovalbumin injections (IP) were performed on days 0 and 7. From days 14, 16, 18, 21 and 24, the animals were exposed to aerosolized OVA or saline. From days 10 to 24 ST were administrated. 72h later, the rats were euthanized.
At days 14, 16, 18, 21, and 24, the rats were placed in a plexiglass exposure chamber connected to the outlet of an ultrason aerosol generator (OMRON, NE-C29-E) for 30min. Ovalbumin (1g OVA in 100mL PBS) (Grade III; Sigma Chemical Co., Poole, UK) challenges were performed with a mean particle size of 3.2μm and with an output of 3mL/min. The last aerosol exposure was done 72h before the end of the experiment13 (Fig. 1). The animals in the other groups were challenged with PBS.
Administration of smokeless tobacco extract (STE)Commercially-prepared ST was bought from the local market (Ets El-Nardjass Company zone 06, Sidi Chehmi-Oran, Algeria). The lethal dose (LD50) concentration of the ST was calculated as 50mg/kg rat body weight (b.w.) using LD50 for nicotine in rats as standard.14 The used ST concentrations were calculated as 80% of the LD50, which is 40mg/kg (b.w.).14
An amount of 1mL from the stock solution was administered by oral gavage (force-feeding) once per day for fifteen (15) days.14 On the days of the challenge, STE was given 30min after the aerosol exposure.8
PlantNSO was obtained from a local commercial market (Ets EL-BARAKA Company BPN°302, Birkadem-Algiers, Algeria). The company produces NSO by cold pressing of fresh seeds without using chemicals. The NSO was administered orally by gavage for 31 days, at a dose of 4mL/kg/day.12 On the days of sensitization and challenge, NSO was given 30min before the treatment.
Experimental protocolThe animals were assigned into eight groups of six rats:
Group 1: Control rats (C); Group 2: Ovalbumin challenged rats (Ova); Group 3: ST exposed rats; Group 4: NSO treated rats; Group 5: Ovalbumin challenged and ST treated rats (Ova/ST); Group 6: Ovalbumin challenged and NSO treated rats (Ova/NSO); Group 7: ST- and NSO-treated rats (ST/NSO); Group 8: Ovalbumin challenged, ST- and NSO-treated rats (Ova/ST/NSO).
Samples preparationAnimals were sacrificed by cervical decapitation. Blood samples were immediately collected in heparin tubes and plain vials, to obtain plasma and serum samples, respectively. Blood samples were centrifuged for 15min at 3000RPM and stored at −20°C prior to use.
The blood sediment after centrifugation containing erythrocytes, which were twice suspended in PBS (pH 7.4) and centrifuged at 3000×g for 15min at 4°C for the first washing; and at 4000×g for 30min at 4°C for the second washing. The lung was quickly removed, washed in 0.9% NaCl solution and weighed after the careful removal of the surrounding connective tissues, and then, about 1g of tissue was homogenized in 2mL of PBS at 1:2 (w/v), in ice-cold condition and pH 7.4. Homogenates were centrifuged at 3000×g for 35min at 4°C. The hemolysates and tissues supernatants were then aliquoted and stored at −20°C prior to use.
The lungs of the rats concerned were lavaged by instillation with 2.0mL PBS. The procedure was repeated three times before lavage fluid was pooled in Eppendorf tubes, and then centrifuged at 4000×g for 10min. Cells were resuspended and enumerated using an improved Neubauer hemocytometer (Full Automatic Blood Cell Counter MODEL PCE-210N). Next, Eppendorf tubes containing BronchoAlveolar Lavage Fluid (BALF) were stored at −20°C prior to use for IL-4, NO and total proteins measurement.
Estimation of lipid peroxidation levelsThe LPO was evaluated using the MalonDiAldehyde (MDA) level as final product in the lung and erythrocytes homogenates. MDA reacts with thiobarbituric acid (TBA) as a reactive substance (TBARS) to produce a red-colored complex.15 The absorbance was read at 530nm.
Reduced glutathione (GSH) levelsGSH contents of lung and erythrocytes homogenates were estimated using a colorimetric technique15 based on the development of a yellow color when DTNB [5,5′-dithiobis-(2nitrobenzoic acid)] was added to compounds containing sulfhydryl groups. The absorbance was recorded at 412nm.
Estimation of antioxidant enzymes activitiesGlutathione peroxidase (GPx) activity was measured at 420nm by the Flohe and Gunzler method.15 Catalase (CAT) activity was measured according to the method of Aebi.15 This assay is based on the ability of the enzyme to induce the disappearance of hydrogen peroxide monitored by following the decrease in the absorbance at 240nm for 1min. Superoxide dismutase (SOD) activity was determined using the method of Beyer and Fridovich.15 It was evaluated by measuring its ability to inhibit the photo-reduction of nitro blue tetrazolium (NBT). The reaction started by switching the light on, and changes in absorbance were recorded at 560nm after 20min.
Protein assaysThe proteins concentrations of supernatants of the lungs, erythrocytes and that of BALF were measured spectrophotometrically at 595nm according to the method of Bradford,15 using bovine serum albumin as standard.
Non-protein thiols (NPSH) determinationNPSH levels were determined by the method of Ellman.16 The supernatant of the lung and erythrocytes was mixed (1:1) with 10% trichloroacetic acid. After centrifugation, the protein pellet was discarded and free –SH groups were determined in the clear supernatant. An aliquot of supernatant was added in 1M potassium phosphate buffer pH 7.4 and 10mM DTNB. The color reaction was measured at 412nm.
Protein carbonyl groups determinationProtein carbonyl groups in the lung and erythrocytes homogenates were estimated by the method of Levine et al.,17 which is based on the derivatization of the carbonyl group with 2,4-dinitrophenylhydrazine (DNPH). This led to the formation of a stable 2,4-dinitrophenyl (DNP) hydrazone product. Absorbance was measured spectrophotometrically at 370nm against a blank of a 6M guanidine solution.
Nitric oxide (NO) measurementsThe NO production in serum and BALF was determined by the detection of nitrite (NO2−) concentration from the Griess reaction.18 The absorbance was measured at 530nm from an automatic microplate reader (Mindray MR-96A). Nitrite concentration was compared to a sodium nitrate standard curve.
Interleukin-4 (IL-4) measurementSerum and BALF IL-4 levels were measured, according to the manufacturer's protocol, using Novex Rat IL-4 ELISA (enzyme-linked immunosorbent assay) commercial kit purchased from Invitrogen (Camarillo, CA, USA). After measuring the optical density at 450nm, the concentrations of IL-4 were determined by interpolation from a standard curve, with all data expressed in pg/mL.
Histopathological examinationPortions of lung were used for histopathological examinations. Tissues were fixed in 10% buffered formalin (pH 7.2) and dehydrated through a series of ethanol solutions, embedded in paraffin, and routinely processed for histological analysis.15 Sections of 5μm in thickness were cut and stained with hematoxylin–eosin for examination. The stained tissues were observed through an optical microscope (LEICA DM-750) and photographed by a digital camera (Canon Elph shot-305).
Statistical analysisValues were presented as means±standard error mean (SEM) for six rats of each group. Significant differences between the group's means were determined by one-way ANOVA followed by Student's t test. The statistical signification of difference was taken as p<0.05.
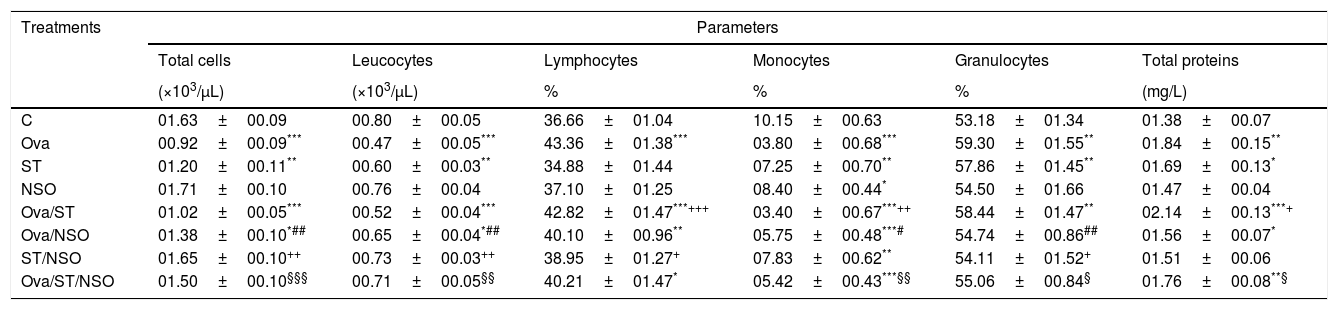
ResultsCell counts and total protein levels in BALFCell counts revealed (in Table 1) that BALF of Ova-sensitized rats or ST contained a significantly decreased number of total cells, leucocytes and monocytes, compared with control animals, and a significant increase in the number of granulocytes. There was a substantial increase in the number of lymphocytes in Ova-sensitized rats. The administration of NSO to sensitized animals resulted in a significant improvement of the total cells, leucocytes, monocytes and granulocytes counts but did not affect the lymphocytes counts.
Cell numbers and total proteins levels in BALF of sensitized and treated rats.
| Treatments | Parameters | |||||
|---|---|---|---|---|---|---|
| Total cells | Leucocytes | Lymphocytes | Monocytes | Granulocytes | Total proteins | |
| (×103/μL) | (×103/μL) | % | % | % | (mg/L) | |
| C | 01.63±00.09 | 00.80±00.05 | 36.66±01.04 | 10.15±00.63 | 53.18±01.34 | 01.38±00.07 |
| Ova | 00.92±00.09*** | 00.47±00.05*** | 43.36±01.38*** | 03.80±00.68*** | 59.30±01.55** | 01.84±00.15** |
| ST | 01.20±00.11** | 00.60±00.03** | 34.88±01.44 | 07.25±00.70** | 57.86±01.45** | 01.69±00.13* |
| NSO | 01.71±00.10 | 00.76±00.04 | 37.10±01.25 | 08.40±00.44* | 54.50±01.66 | 01.47±00.04 |
| Ova/ST | 01.02±00.05*** | 00.52±00.04*** | 42.82±01.47***+++ | 03.40±00.67***++ | 58.44±01.47** | 02.14±00.13***+ |
| Ova/NSO | 01.38±00.10*## | 00.65±00.04*## | 40.10±00.96** | 05.75±00.48***# | 54.74±00.86## | 01.56±00.07* |
| ST/NSO | 01.65±00.10++ | 00.73±00.03++ | 38.95±01.27+ | 07.83±00.62** | 54.11±01.52+ | 01.51±00.06 |
| Ova/ST/NSO | 01.50±00.10§§§ | 00.71±00.05§§ | 40.21±01.47* | 05.42±00.43***§§ | 55.06±00.84§ | 01.76±00.08**§ |
Values are given as mean±SEM for groups of six animals each. Significant difference: all treated groups compared to the control one (*p<0.05, **p<0.01, ***p<0.001), compared to the Ova sensitized one (#p<0.05, ##p<0.01), compared to the ST treated one (+p<0.05, ++p<0.01, +++p<0.001), compared to the Ova/ST treated one (§p<0.05, §§p<0.01, §§§p<0.001).
One-way ANOVA showed a significant increase in BALF total proteins levels in Ova-sensitized, ST exposed and Ova/ST co-exposure compared to the control group. The administration of NSO revealed a significant improvement only in combined Ova/ST exposed rats (Table 1).
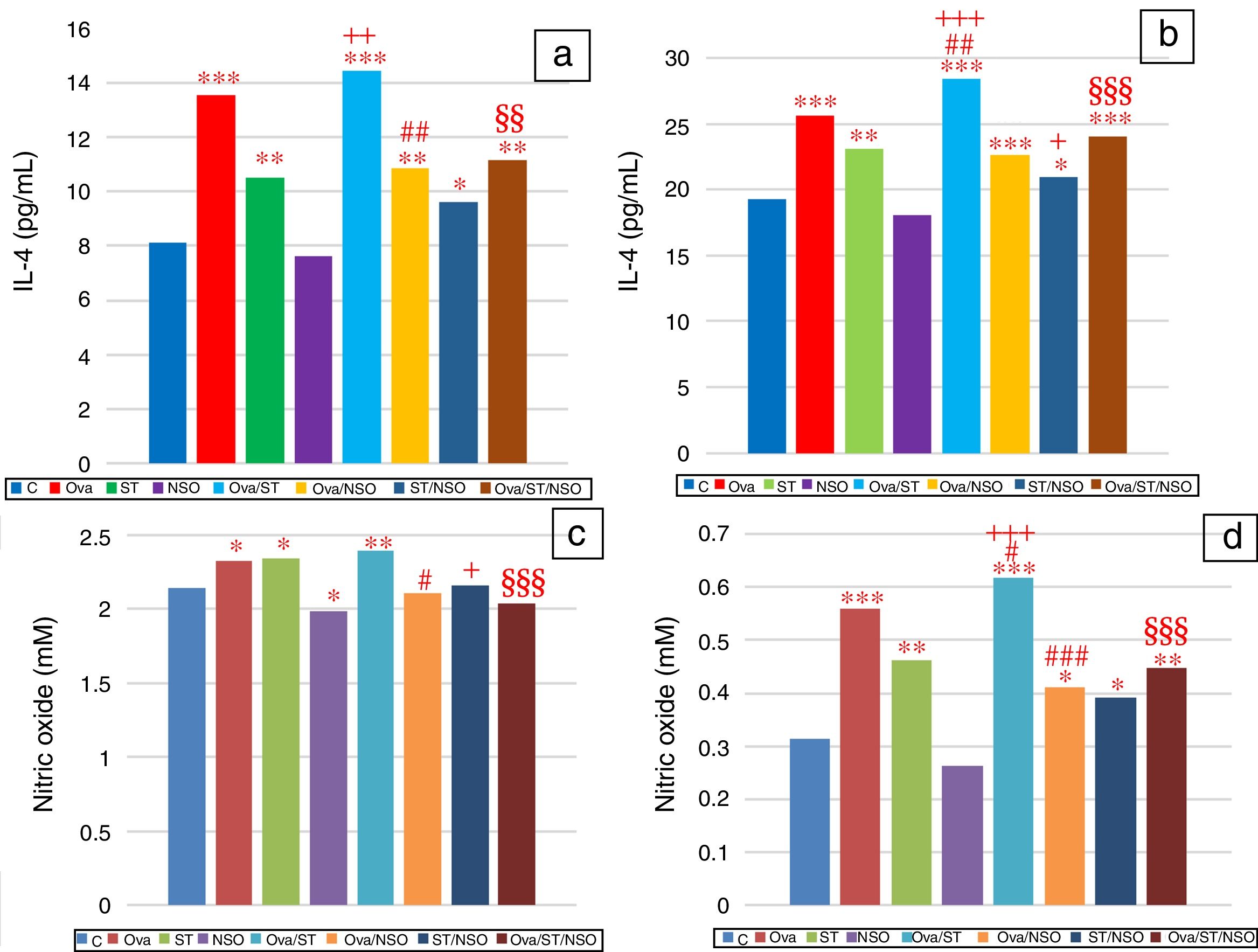
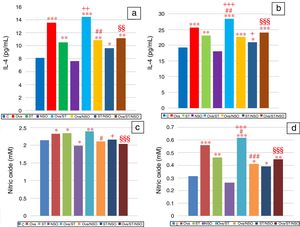
IL-4 levels in serum and BALFSerum IL-4 levels indicated a significant increase in Ova-sensitized, ST treated and Ova and ST co-exposed rats compared to control rats (Fig. 2a). However, the administration of NSO to sensitized animals resulted in a significant improvement of IL-4 levels in Ova-sensitized group and Ova/ST co-exposed group.
Significant difference: all treated groups compared to the control one (*p<0.05, **p<0.01, ***p<0.001), compared to the Ova sensitized one (#p<0.05, ##p<0.01, ###p<0.001), compared to the ST treated one (+p<0.05, ++p<0.01, +++p<0.001), compared to the Ova/ST treated one (§§p<0.01, §§§p<0.001).
BALF IL-4 levels also showed a significant increase in Ova-sensitized, ST treated and Ova and ST co-exposed rats compared to control rats (Fig. 2b). In addition, combined Ova and ST exposure showed a significant increase compared to Ova-sensitized and ST exposed rats. However, the administration of NSO to sensitized animals resulted in a significant improvement of IL-4 levels in Ova-sensitized group, ST exposed group and Ova/ST co-exposed group.
NO levels in serum and BALFSerum NO levels revealed a considerable increase in Ova-sensitized, ST exposed and Ova/ST co-exposed rats and a significant decrease in NSO-treated group compared to the control group (Fig. 2c). However, NSO administration resulted in a significant improvement in Ova-sensitized, ST exposed and Ova/ST co-exposed rats.
BALF NO levels showed a significant increase in Ova-sensitized, ST exposed and Ova/ST co-exposed rats compared to the control group (Fig. 2d). Nevertheless, NSO administration resulted in a significant improvement in Ova-sensitized, ST exposed and Ova/ST co-exposed rats.
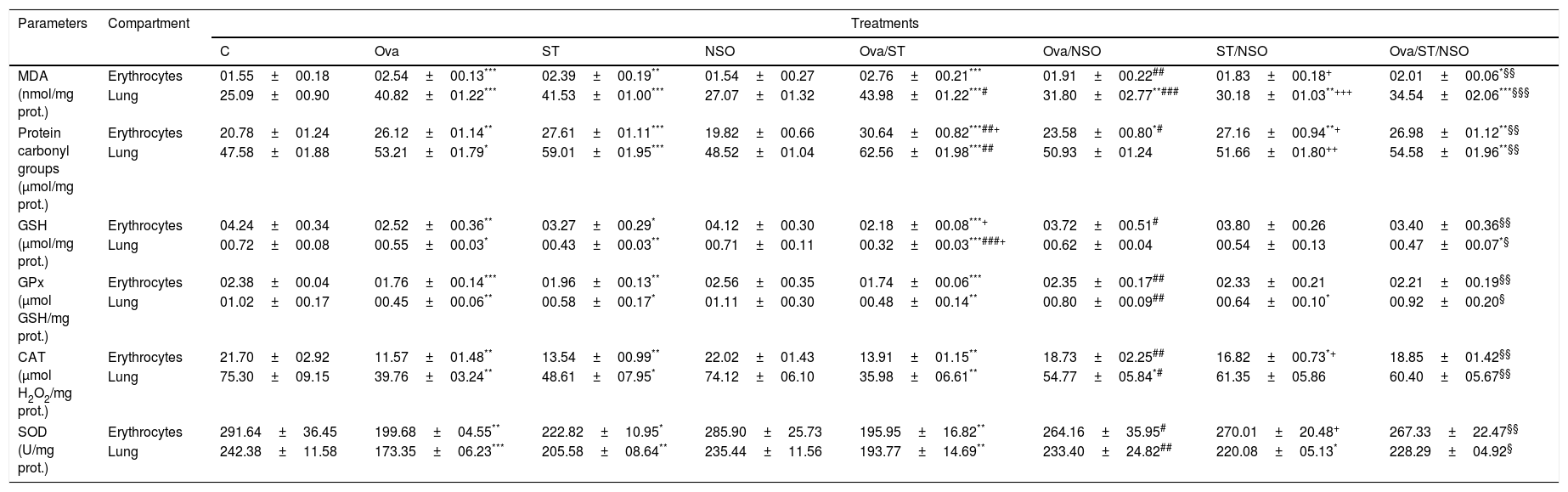
Antioxidant defense status in the lung and erythrocytesOva-sensitization, ST exposure and Ova/ST co-exposure considerably increased the levels of MDA and protein carbonyl groups and decreased levels of GSH, GPx, CAT and SOD in the lung and erythrocytes of the sensitized group compared with the control group. Moreover, NSO administration resulted in a significant improvement of all these parameters in the studied organs (Table 2).
Antioxidant defense status in lung and erythrocytes of treated and control rats.
| Parameters | Compartment | Treatments | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Ova | ST | NSO | Ova/ST | Ova/NSO | ST/NSO | Ova/ST/NSO | ||
| MDA (nmol/mg prot.) | Erythrocytes | 01.55±00.18 | 02.54±00.13*** | 02.39±00.19** | 01.54±00.27 | 02.76±00.21*** | 01.91±00.22## | 01.83±00.18+ | 02.01±00.06*§§ |
| Lung | 25.09±00.90 | 40.82±01.22*** | 41.53±01.00*** | 27.07±01.32 | 43.98±01.22***# | 31.80±02.77**### | 30.18±01.03**+++ | 34.54±02.06***§§§ | |
| Protein carbonyl groups (μmol/mg prot.) | Erythrocytes | 20.78±01.24 | 26.12±01.14** | 27.61±01.11*** | 19.82±00.66 | 30.64±00.82***##+ | 23.58±00.80*# | 27.16±00.94**+ | 26.98±01.12**§§ |
| Lung | 47.58±01.88 | 53.21±01.79* | 59.01±01.95*** | 48.52±01.04 | 62.56±01.98***## | 50.93±01.24 | 51.66±01.80++ | 54.58±01.96**§§ | |
| GSH (μmol/mg prot.) | Erythrocytes | 04.24±00.34 | 02.52±00.36** | 03.27±00.29* | 04.12±00.30 | 02.18±00.08***+ | 03.72±00.51# | 03.80±00.26 | 03.40±00.36§§ |
| Lung | 00.72±00.08 | 00.55±00.03* | 00.43±00.03** | 00.71±00.11 | 00.32±00.03***###+ | 00.62±00.04 | 00.54±00.13 | 00.47±00.07*§ | |
| GPx (μmol GSH/mg prot.) | Erythrocytes | 02.38±00.04 | 01.76±00.14*** | 01.96±00.13** | 02.56±00.35 | 01.74±00.06*** | 02.35±00.17## | 02.33±00.21 | 02.21±00.19§§ |
| Lung | 01.02±00.17 | 00.45±00.06** | 00.58±00.17* | 01.11±00.30 | 00.48±00.14** | 00.80±00.09## | 00.64±00.10* | 00.92±00.20§ | |
| CAT (μmol H2O2/mg prot.) | Erythrocytes | 21.70±02.92 | 11.57±01.48** | 13.54±00.99** | 22.02±01.43 | 13.91±01.15** | 18.73±02.25## | 16.82±00.73*+ | 18.85±01.42§§ |
| Lung | 75.30±09.15 | 39.76±03.24** | 48.61±07.95* | 74.12±06.10 | 35.98±06.61** | 54.77±05.84*# | 61.35±05.86 | 60.40±05.67§§ | |
| SOD (U/mg prot.) | Erythrocytes | 291.64±36.45 | 199.68±04.55** | 222.82±10.95* | 285.90±25.73 | 195.95±16.82** | 264.16±35.95# | 270.01±20.48+ | 267.33±22.47§§ |
| Lung | 242.38±11.58 | 173.35±06.23*** | 205.58±08.64** | 235.44±11.56 | 193.77±14.69** | 233.40±24.82## | 220.08±05.13* | 228.29±04.92§ | |
Values are given as mean±SEM for groups of six animals each. Significant difference: all treated groups compared to the control one (*p<0.05, **p<0.01, ***p<0.001), compared to the Ova sensitized one (#p<0.05, ##p<0.01, ###p<0.001), compared to the ST treated one (+p<0.05, ++p<0.01, +++p<0.001), compared to the Ova/ST treated one (§p<0.05, §§p<0.01, §§§p<0.001).
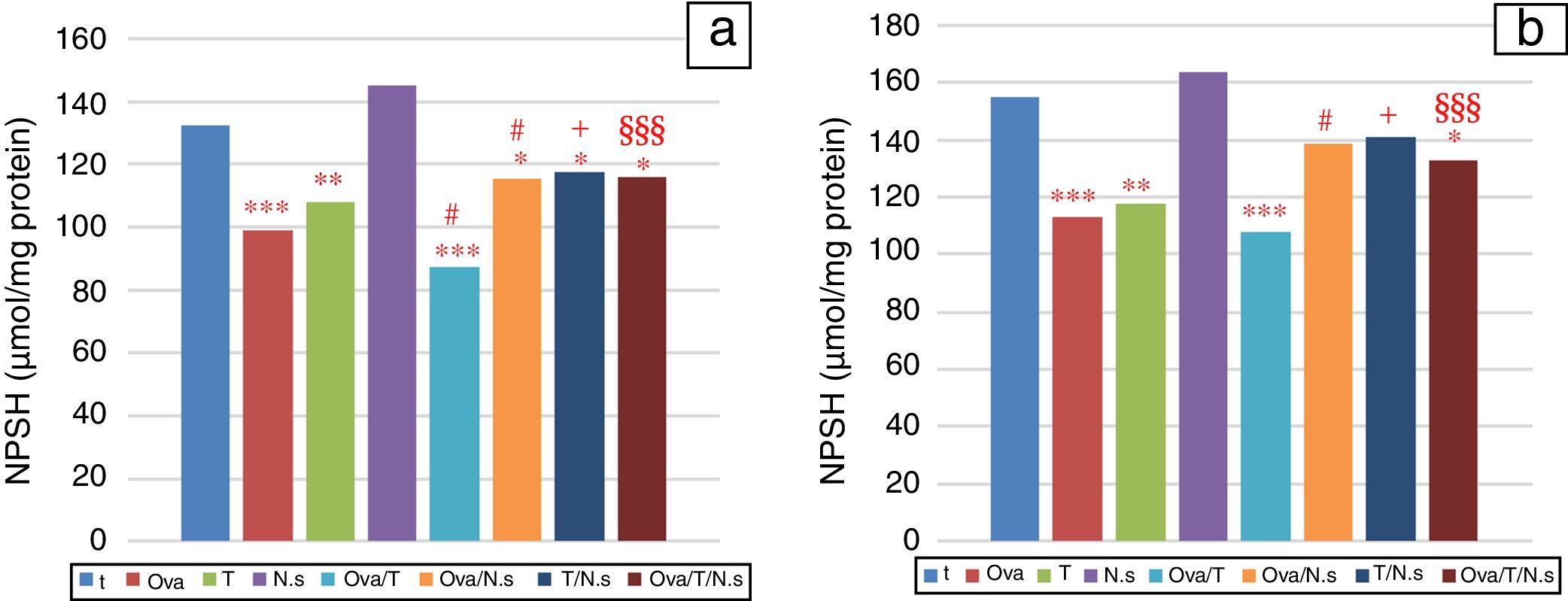
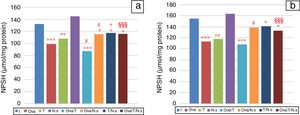
NPSH levels showed a significant decrease in Ova-sensitized, ST-exposed and Ova/ST-co-exposed rats compared to the control group in the lung and erythrocytes (Fig. 3). However, NSO administration brought about an important improvement in Ova-sensitized, ST-exposed and Ova/ST co-exposed rats.
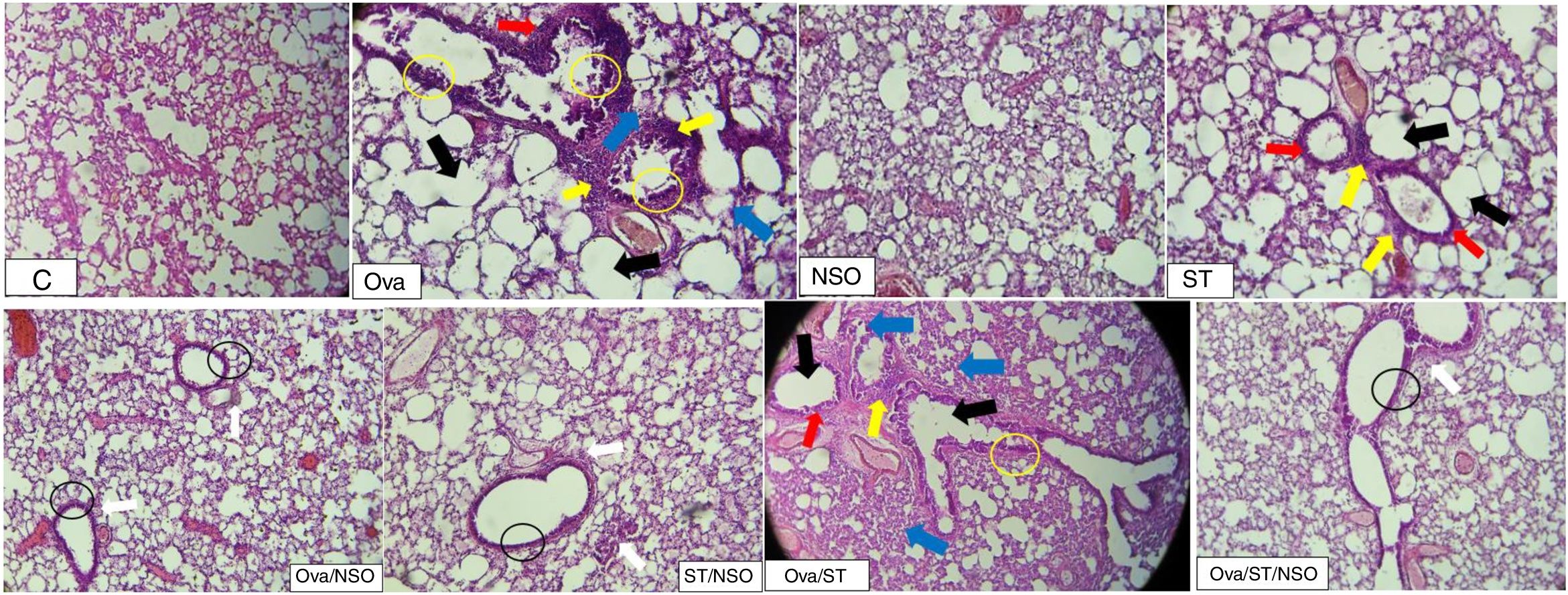
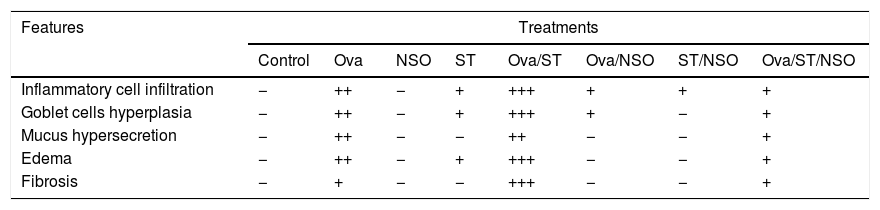
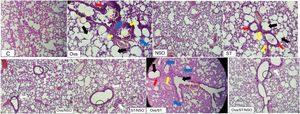
Histopathological resultsThe histopathological examination of Ova-sensitized, ST-exposed and Ova/ST-co-exposed rats’ lung revealed an inflammatory cell infiltration (yellow arrow), goblet cells hyperplasia (red arrow) with mucus hypersecretion (yellow circle) edema (black arrow) and fibrosis (blue arrow) (Fig. 4). However, NSO administration revealed a reduction of inflammatory cell infiltration (white arrow), a smaller degree of goblet cells hyperplasia (dark circle) and normal cells morphology compared to Ova-sensitized, ST-exposed and Ova/ST-co-exposed groups. Furthermore, no histological alterations were observed in the lung of NSO-treated group when compared to the control (Table 3).
The control rat showing normal histological structure. Ova-sensitized, ST exposed and combined Ova/ST exposed rats’ lung showing inflammatory cell infiltration (yellow arrow), goblet cells hyperplasia (red arrow), mucus hypersecretion (yellow circle), edema (black arrow) and fibrosis (blue arrow). The NSO-treated rat lung showing reduction of inflammatory cell infiltration (white arrow), a smaller degree of goblet cells hyperplasia (dark circle) and normal cells morphology compared to the control group. NSO-treated rat lung showing normal appearance of cells.
Semi-quantitative scoring of architectural damage on histopathological examination of the rat lungs in the different treatment groups.
| Features | Treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Ova | NSO | ST | Ova/ST | Ova/NSO | ST/NSO | Ova/ST/NSO | |
| Inflammatory cell infiltration | − | ++ | − | + | +++ | + | + | + |
| Goblet cells hyperplasia | − | ++ | − | + | +++ | + | − | + |
| Mucus hypersecretion | − | ++ | − | − | ++ | − | − | + |
| Edema | − | ++ | − | + | +++ | − | − | + |
| Fibrosis | − | + | − | − | +++ | − | − | + |
(−) indicates none or without features, (+) indicates mild, (++) indicates moderate and (+++) indicates severe.
It has been well proven that cigarette smoke causes the exacerbation of asthma as demonstrated by functional airway hyperreactivity and elevated levels of blood eosinophilia in Ova-sensitized mice.8 However, to the best of our knowledge, no study is available showing comparisons of ST exposure versus Ova-sensitized rats or asthmatic patients. In the present study, we selected an animal model of asthma showing all the salient features of allergic airway inflammation in humans, namely increased Th2 cytokine levels in BALF, enhanced airway responsiveness, airway eosinophilic inflammation, and goblet cell hypertrophy and hyperplasia.12 Under these conditions, IL-4 is necessary for the differentiation of T cells to the Th2 type. It is also a key factor for isotype switching to IgE in B cells,19 regulation of chemokines required for eosinophil migration,20 and may increase mucus secretion in the allergic airways.13 In fact, Mauser et al.21 suggested that the provocation with the allergen (in an animal model of asthma) induced increased microvascular infiltration and edema, thus the swelling of the inflamed organ. This inflammatory phenomenon, which is due to the penetration of the allergen (like Ovalbumin), results in the migration of inflammatory cells from the vascular compartment to the lungs. The ratio of leukocytes, attested by cytologic examination of blood and fluid of bronchoalveolar lavage, increase and represent an important regulator of the immune response. Thus, the migration of these inflammatory cells (especially the eosinophils) in the lungs, also attested by histopathological analysis of lung tissues, mainly contributes to the development of airway inflammation that induces epithelial damage leading to bronchial remodeling.22
The results obtained revealed that Ova/ST co-exposure significantly increased IL-4 and NO levels in BALF when compared to Ova-sensitized rats. However, NSO administration showed a significant improvement compared to Ova/ST co-exposed rats. Indeed, NO is a signaling molecule responsible for several diverse physiological and pathophysiological processes. Up to now, the prevailing hypothesis about NO has been that it contributes to toxicant-induced lung inflammation and injury.23 Many previous studies have associated the expression of NO with patients suffering from asthma24 and those with Ova-induced asthma in experimental animals25 with an elevated degree with cigarette smokers having this disease.26 Moreover, previous studies27 have corroborated that components within ST extract can influence the course of T cell cytokine production.
However, Boskabady et al.28 have reported that the hydroethanolic extract of N. sativa seeds suppresses IL-4 production and enhances IFN-γ production in the blood of Ova-sensitized guinea pigs. Moreover, Balaha et al.12 clearly demonstrated that the oral treatment with NSO dose dependently inhibited Th2 cytokine production (IL-4, IL-5 and IL-13) and recovered the decreased Th1 cytokine production (IFN-γ) in the BALF after antigen challenge in sensitized mice. Furthermore, as reported in the literature, the production of NO is also influenced by the presence of different alkaloids (nigellidine, nigellimine and nigellicine) in NSO.25
NO is a free radical playing a pivotal role just as much as the LPO. It is one of the main manifestations of oxidative damages, which has proven to play an important role in the toxicity and carcinogenicity of several xenobiotics.29 Moreover, protein carbonyls have a major advantage over lipid peroxidation products as oxidized proteins are generally more stable, form earlier and circulate in the blood for longer periods.30 Actually, it is the most general indicator and by far the most commonly used marker of protein oxidation and the accumulation of protein carbonyls. The latter has been observed in several human diseases, including inflammatory and oxidative lung injury.31 In the present study, the levels of malondialdehyde were measured to indicate the generation of ROS and tissue damage induced by the LPO in erythrocytes and lungs. We observed significantly high MDA levels in the lung of Ova/ST co-exposed rats compared to the Ova-sensitized group. This accumulation is an indicator of peroxidation of microsomal lipids.32 Furthermore, it has been shown that ST administration in rats induces oxidative stress resulting in the boosted levels of MDA,3 as well as in ST human consumers.2 Moreover, we noticed a significant increase of protein carbonyl content in the erythrocytes and the lung of Ova/ST co-exposed rats compared to the Ova-sensitized group. The intracellular level of oxidized proteins reflects the balance between the rate of protein oxidation and that of oxidized protein degradation.33 However, NSO supplementation causes a significant decrease of MDA levels and protein carbonyl content in Ova/ST co-exposed rats. This improvement could be explained by the fact that NSO possesses components such as polyphenols and flavonoids that prevent the formation of ROS.34 It has also been demonstrated that NSO enhances the tissue capacity for detoxifying ROS.35
On the other hand, reducing glutathione is only one among many potential antioxidant defenses involved in the protection of various organs against oxidant-induced injury in inflammation.36 Therefore, GSH is a strong nucleophile that often inactivates electrophilic reactive compounds by either direct non-enzymatic conjugation or enzymatic catalysis.3 During the metabolism of tobacco, many electrophiles which are detoxified by the use of GSH are generated.37 The decreased GSH levels increase the free radical burden due to ineffective removal of ROS from the tissues. Our results proved that Ova/ST co-exposure significantly decreased GSH levels and GPx activity in lung compared to the Ova-sensitized group. Thus, the toxicity of ST in various organs – especially the lung – might be due to the formation of the radical species. The overproduction of these radicals has an inhibitory effect on the enzymes responsible for the removal of ROS, such as GPx.3 Thus, its decrease is the reflection of its intense use in both situations of cellular stress, namely those of allergic inflammation and tobacco administration. Nonetheless, NSO pretreatment in Ova/ST co-exposed rats resulted in a significant improvement of GSH levels in lung, which suggests that NSO may be protective against the oxidation via free-radical scavenging property. Indeed, it has previously been reported in the literature that phytochemicals stimulate the synthesis of antioxidant enzymes and detoxification systems at the transcriptional level through antioxidant response elements.38 The pre-treatment with NSO, in the present study, significantly increased GPx activity in Ova, ST- and Ova/ST-exposed rats. These findings extended the knowledge of the antioxidant effects of NSO extracts, which is certainly due to their content of thymoquinone.39
Among the antioxidants found in the case of stress, non-protein thiol is present in high concentrations because it plays a crucial role in protecting the lung from oxidative stress by detoxifying exogenous and endogenous toxicants, and quenching ROS.40 In this study, the levels of NPSH were found to significantly decrease in the lungs of Ova/ST co-exposed rats compared to the Ova-sensitized rats. Indeed, various experimental research studies41 have demonstrated that tobacco exposure induces the depletion of NPSH in the lungs of rats. However, NSO administration resulted in the significant increase of NPSH in Ova, ST and Ova/ST exposed rats. The findings are in agreement with many previous studies that have demonstrated that supplementation with antioxidants such as diphenyl diselenide,42 selenium43 and rutin44 leads to the restoration of NPSH levels in oxidative stress injury.
All previous results are in accordance with the present histopathological study of lung. In fact, the contraction, thickening and abnormality of the smooth muscle of airways are mainly responsible for the AHR in asthma. The lung histopathological finding of this study showed inflammatory cell infiltration, goblet cells hyperplasia and mucus hypersecretion in the OVA and OVA/ST treated rats. The release of various inflammatory mediators like IL-4 has been confirmed to play a vital role in mucus hypersecretion and goblet cell hyperplasia.22
Over a hundred different chemical constituents have been identified in N. sativa, attributing it therapeutic virtues. The black cumin seed is composed of fixed (stable) and essential (volatile) oil which is responsible for many of the beneficial effects. The fixed oil contains appreciable quantities of unsaturated fatty acids (linoleic, oleic, and inolenic acids) as well as saturated fatty acids in minor amounts (arachidonic and eicosenoic acids), all considered as powerful antioxidants.7,9 In addition to the fatty acid profile, other bioactive compounds have been reported, such as vitamin E (tocopherol α, β, and γ), retinol (vitamin A), carotenoids (β-carotene), and thymoquinone (2-isopropyl-5methyl-1,4-benzoquinone), fat-soluble vitamins. Some of the phytosterols which have a high beneficial effect on inflammation include β-sitosterol, avenasterol, stigmasterol, campesterol and TQ, 46% monoterpenes including p-cymene, α-pinene, thymol (THY), dithymoquinone (DTQ, nigellone), and thymohydroquinone (THQ).9
Finally, in harmony with the literature data showing the anti-inflammatory and antioxidant effects of NS,12 the present study validates not only antiasthmatic traditional use of the N. sativa but in addition showed that NSO administration deleted the proinflammatory and oxidative stress changes caused either by OVA sensitization or ST administration or both at once. Actually, this is what offers the quality of originality to our study. Thanks to its well-documented anti-inflammatory and antioxidant properties N. sativa would protect against the adverse effects of ST and is more endowed with anti-asthmatic properties.
ConclusionOverall, the findings of the present study demonstrated that the short-term administration of ST in a rat model of allergic asthma clearly contributed to the worsening of lung inflammation through the increase in the production of IL-4 and NO. Moreover, ST intensifies the oxidative stress state induced by Ova-challenge in rat, which was shown by augmenting lipid peroxidation, protein oxidation and by altering the non-enzymatic and enzymatic antioxidant status. Furthermore, the aggravation of inflammation and oxidative stress was obviously affirmed by the lung histopathological changes observed in the present study. In contrast, the NSO administration has shown anti-inflammatory and antioxidant effects by reducing IL-4 and NO production, restoration of the antioxidant status and reduction of lipid peroxidation and protein oxidation. Besides, the NSO treatment improved the histological alterations induced by Ova and ST co-exposure in rats.
Conflict of interestThe authors have no conflict of interest to declare.
The authors would like to thank the DGSRTD (General Directorate for Scientific Research and Technological Development) and the ATRSS (Agence Thématique de Recherche en Sciences de la Santé) for the support of this research work, via PNR projects (33/DFPR/ATRSS). They also wish to extend their thanks to Mrs. Leila MAHFOUDHI, Major teacher of English at the Sfax Faculty of Science, for proofreading and polishing the language of the manuscript.