PCA3 performance as a single second line biomarker is compared to the European Randomized Study of Screening for Prostate Cancer risk calculator model 3 (ERSPC RC-3) in an opportunistic screening in prostate cancer (PCa).
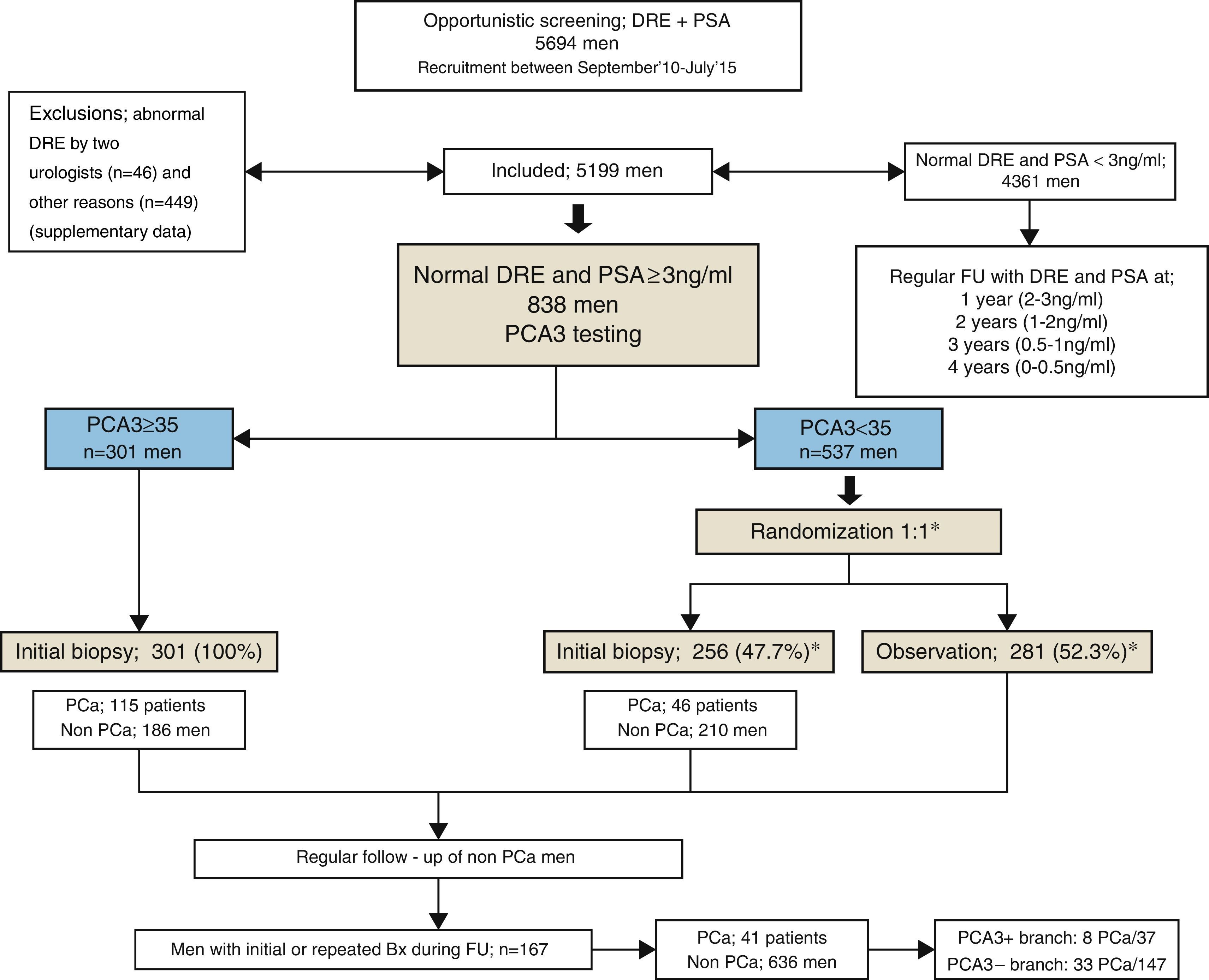
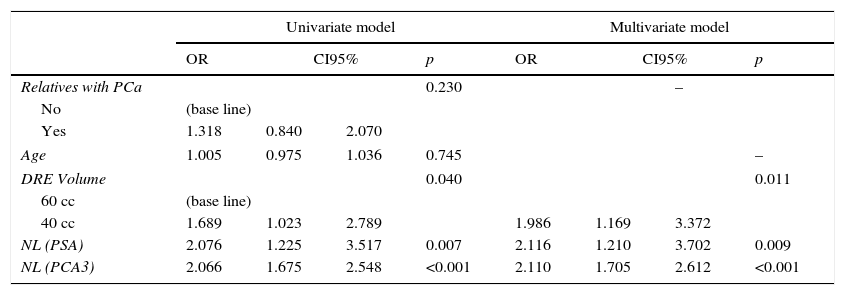
Material and methods5199 men, aged 40–75 years, underwent prostate-specific antigen (PSA) screening and digital rectal examination (DRE). Men with a normal DRE and PSA ≥3ng/ml had a PCA3 test done. All men with PCA3 ≥35 underwent an initial biopsy (IBx) – 12 cores. Men with PCA3 <35 were randomized 1:1 to either IBx or observation. We compared them to those obtained with ERSPC RC-3.
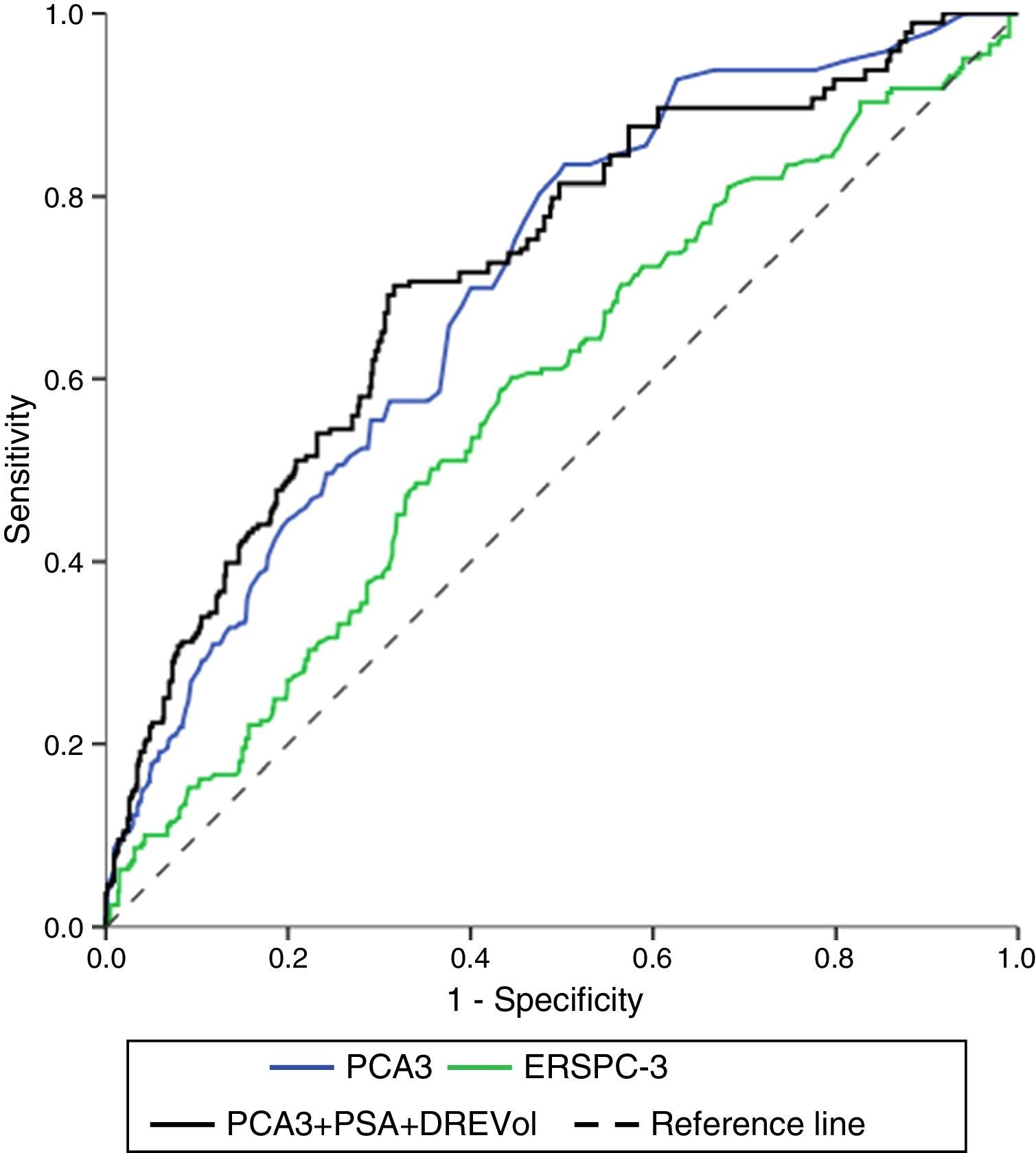
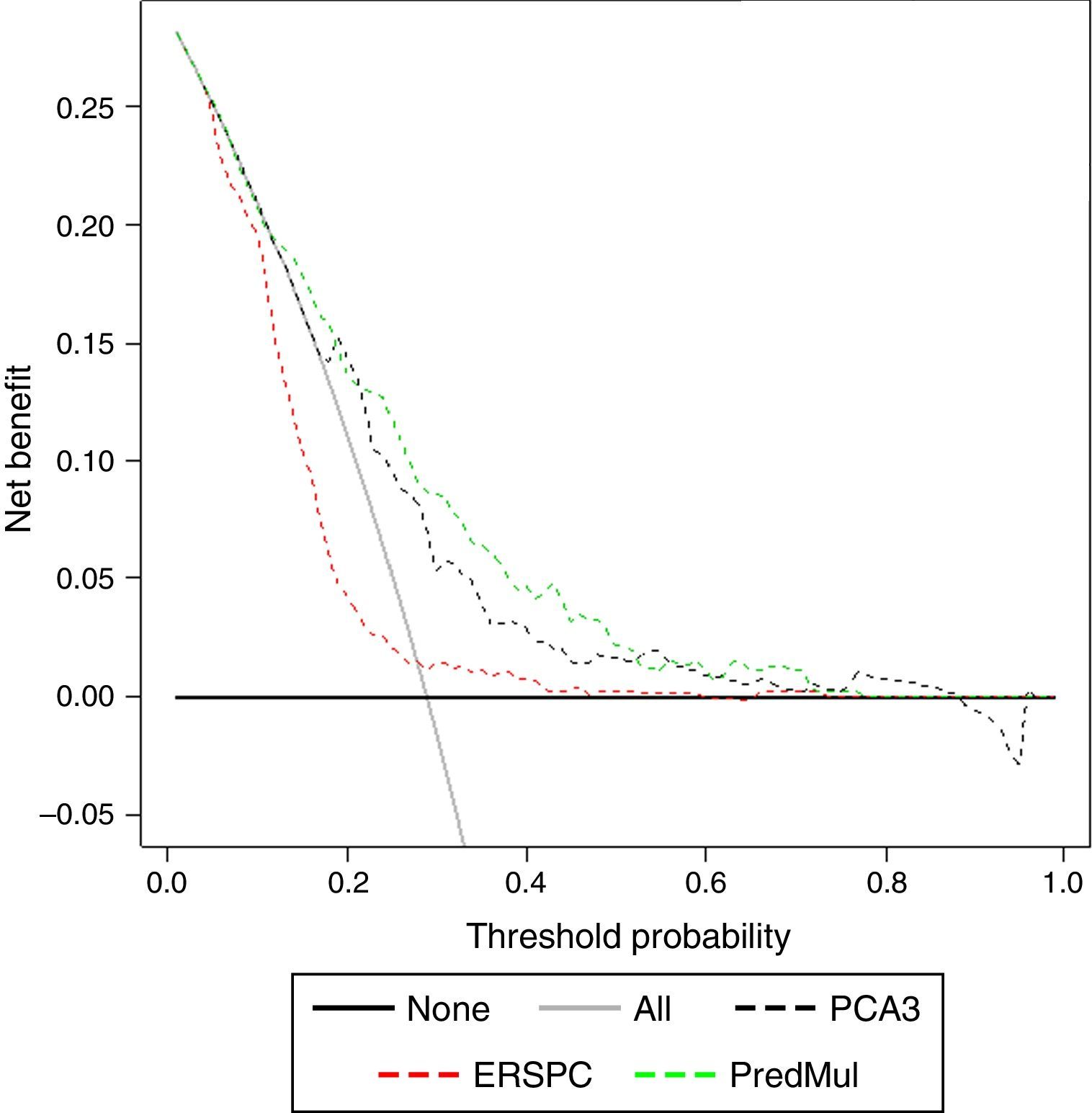
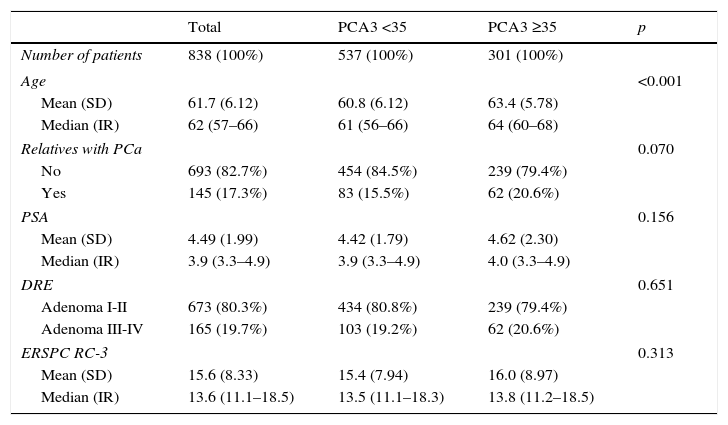
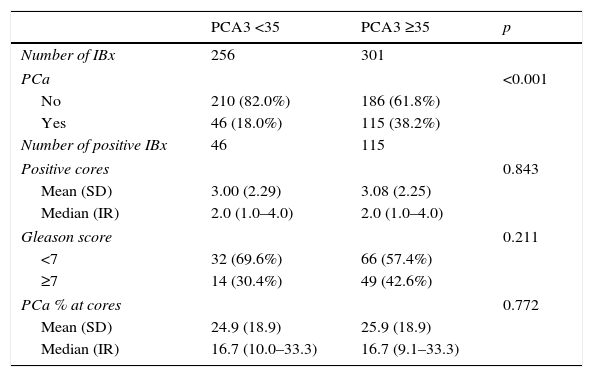
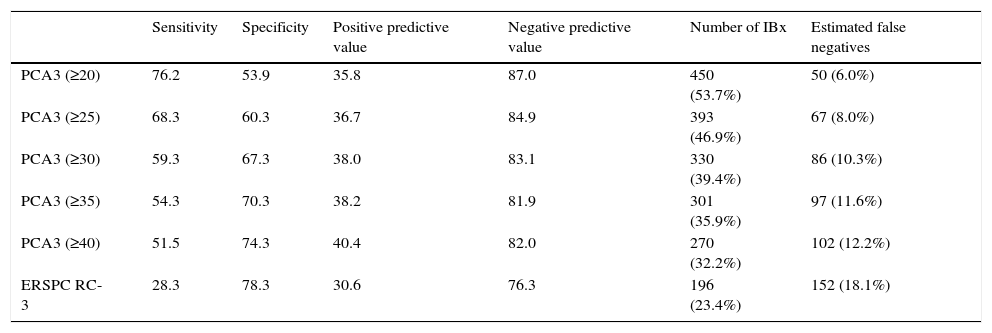
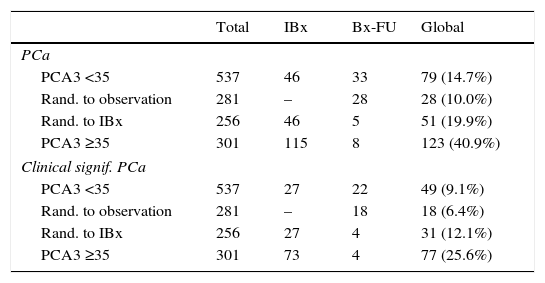
ResultsPCA3 test was performed on 838 men (16.1%). In PCA3(+) and PCA3(–) groups, global PCa detection rates were 40.9% and 14.7% with a median follow-up (FU) of 21.7 months (p<0.001). In the PCA3(+) arm (n=301, 35.9%), PCa was identified in 115 men at IBx (38.2%). In the randomized arm, 256 underwent IBx and PCa was found in 46 (18.0%) (p<0.001). The biopsy-sparing potential would have been 64.1% as opposed to 76.6% if we had used ERSPC RC-3. However, the estimated false negative cases for HGPCa would have been reduced by 37.1% (89–56 patients). Moreover, if we had applied PCA3 -35 to avoid IBx, 14.7% PCa and 9.1% of clinical significant PCa patients would not have been diagnosed during this FU.
ConclusionsWhen PCA3 -35 is used as a second-line biomarker when PSA ≥3ng/ml and DRE is normal, IBx could be avoided in 12.5% less than if ERSPC RC-3 is used and would reduce the false negative cases by 36.2%. At a FU of 21.7 months, this dual protocol would miss 9.1% of clinically significant PCa, so strict FU is mandatory with established biopsy criteria based on PSA and DRE in cases with PCA3 <35.
Determinar el comportamiento del PCA3 como un marcador de segunda línea en un programa de cribado oportunista de cáncer de próstata (CaP) y su comparación con la calculadora de riesgo 3 del cribado aleatorizado europeo en cáncer de próstata (ERSPC RC-3).
Material y métodosUn total de 5.199 hombres de 40-75 años se hicieron la prueba del antígeno prostático específico (PSA) y un tacto rectal (TR). Aquellos con TR normal y PSA ≥3ng/ml se realizaron un PCA3. Todos los hombres con PCA3 ≥35 se hicieron biopsia inicial (BxI) —12 cilindros—. Aquellos con PCA3 <35 se aleatorizaron 1:1 a BxI u observación. Los resultados se comparan con los obtenidos con la aplicación de la calculadora ERSPC RC-3.
ResultadosPCA3 se testó en 838 hombres (16,1%). En los grupos PCA3(+) y PCA3(–), las tasas de detección global de CaP fueron del 40,9 y del 14,7% a una mediana de seguimiento de 21,7 meses (p<0,001. En el grupo PCA3(+) (n=301, 35,9%), se identificó CaP en 115 hombres en BxI (38,2%). En el brazo aleatorizado, 256 se hicieron BxI y se objetivó CaP en 46 (18,0%) (p<0,001). La potencial tasa de ahorro de biopsias siguiendo el corte PCA3=35 hubiera sido de 64,1% frente a la de 76,6% si hubiéramos usado ERSPC RC-3. Sin embargo, la tasa estimada de falsos negativos de CaP de alto grado (CaPAG=Gleason ≥7) se hubiera reducido un 37,1% (de 89 a 56 pacientes) al usar el PCA3. Si hubiéramos usado el corte 35 de PCA3 para no realizar BxI, hubiésemos dejado de diagnosticar un 14,7% de CaP y un 9,1% de CaP clínicamente significativo, a un seguimiento medio aproximado de 2 años.
ConclusionesCuando se usa PCA3-35 como biomarcador de segunda línea en hombres con PSA ≥3ng/ml y TR normal, se puede obviar la BxI un 12,5% menos que usando la ERSPC RC-3, pero reduciendo los falsos negativos un 36,2%. A un seguimiento de 21,7 meses, este protocolo dual no hubiera detectado un 9,1% de CaP clínicamente significativo, por lo que el seguimiento con estrictos criterios de biopsia basados en PSA y TR es obligatorio en casos con PCA3 <35.