Transrectal ultrasound-guided prostate biopsy (TUPB) is associated with infectious complications (ICs), which are related to a greater prevalence of ciprofloxacin-resistant bacteria (CRB) in rectal flora. We examined the ICs that occurred in 2 groups: A guided antibiotic prophylaxis (GP) group and an empiric prophylaxis (EP) group. We assessed the financial impact of GP.
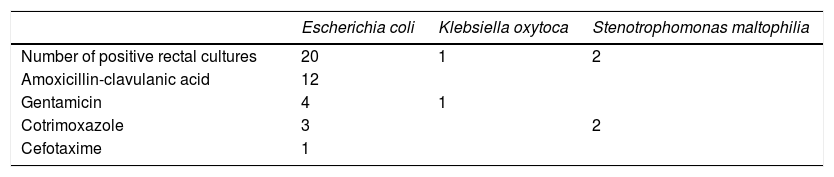
Material and methodsThe GP group was studied prospectively (June 2013 to July 2014). We collected rectal cultures (RCs) before the TUPB, which were seeded on selective media with ciprofloxacin to determine the presence of CRB. The patients with sensitive bacteria were administered ciprofloxacin. Patients with resistant bacteria were administered GP according to the RC antibiogram.
The EP group was studied retrospectively (January 2011 to June 2009). RCs were not performed, and all patients were treated with ciprofloxacin as prophylaxis.
The ICs in both groups were recorded during a period no longer than 30 days following TUPB (electronic medical history).
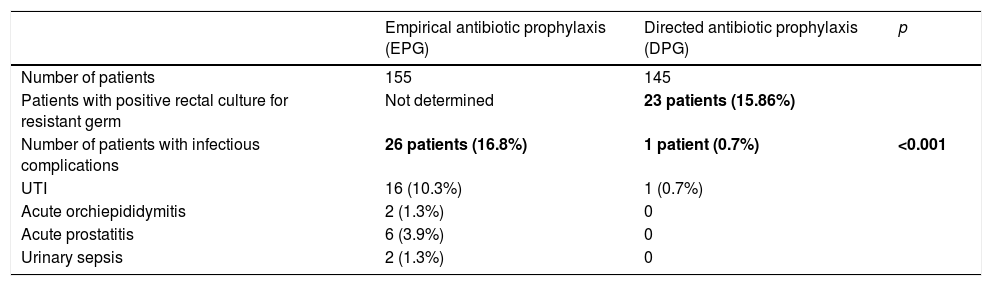
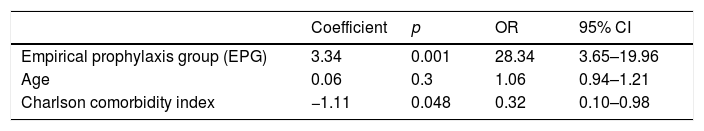
ResultsThree hundred patients underwent TUPB, 145 underwent GP, and 155 underwent EP. In the GP group, 23 patients (15.86%) presented CRB in the RCs. Only one patient (0.7%) experienced a UTI. In the EP group, 26 patients (16.8%) experienced multiple ICs (including 2 cases of sepsis) (p<.005). The estimated total cost, including the management of the ICs, was €57,076 with EP versus €4802.33 with GP. The average cost per patient with EP was €368.23 versus €33.11 with GP. GP achieved an estimated total savings of €52,273.67. Six patients had to undergo GP to prevent an IC.
ConclusionsGP is associated with a marked decrease in the incidence of ICs caused by CRB and reduced healthcare costs.
La biopsia prostática transrectal ecográficamente dirigida (BPTE) se asocia a complicaciones infecciosas (CI). Las CI están relacionadas con un incremento de la prevalencia de bacterias ciprofloxacino-resistentes (BCR) en la flora rectal. Estudiamos las CI ocurridas en 2 grupos. Grupo de profilaxis antibiótica «dirigida» (GPD) vs. grupo de profilaxis empírica (GPE). Evaluamos el impacto económico que supone la profilaxis antibiótica «dirigida» (PD).
Material y métodosEl GPD se estudió prospectivamente (junio 2013-julio 2014). Se recogieron cultivos rectales (CR) antes de BPTE y se sembraron en medios selectivos con ciprofloxacino para determinar la presencia de BCR. Los pacientes con bacterias sensibles recibieron ciprofloxacino. Pacientes con bacterias resistentes recibieron PD según antibiograma del CR.
El GPE se estudió retrospectivamente (enero 2011-junio 2009). El CR no se realizó y todos los pacientes recibieron ciprofloxacino como profilaxis.
Las CI ocurridas en ambos grupos se registraron en un periodo no superior a 30 días después de BPTE (historia clínica electrónica).
ResultadosTrescientos pacientes fueron sometidos a BPTE, 145 recibieron PD y 155 PE. En el GPD, 23 pacientes (15,86%) presentaron BCR en CR. Solo un paciente (0,7%) experimentó ITU. En el GPE, 26 pacientes (16,8%) experimentaron múltiples CI (incluidas 2 sepsis) (p<0,005). El coste total estimado, incluido el manejo de las CI, fue de 57.076€ con PE vs. 4.802,33€ con PD. El coste promedio/paciente con PE fue de 368,23€ vs. 33,11€ con PD. La PD logró un ahorro total estimado de 52.273,67€. Es necesario que 6 pacientes se sometan a PD para prevenir una CI.
ConclusionesLa PD se asoció a un notable descenso de la incidencia de CI causadas por BCR y redujo los costos de atención sanitaria.